Journal Name: Journal of Cardiovascular Disease and Medicine
Article Type: Research
Received date: 03 March, 2020
Accepted date: 24 March, 2020
Published date: 26 March, 2020
Citation: Kurishima C, Inuzuka R, Iwamoto Y, Vinh D, Ishido H, Masutani S, Senzaki H (2020) Effect of Fenestration on Fontan Hemodynamics: Theoretical Analysis Based on a Computational Model of Fenestrated Fontan Circulation. J Cardiovasc Dis Med. Vol: 3, Issu: 1 (05-12).
Copyright: Copyright: © 2020 Kurishima C et al. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Fontan procedure is a palliative surgery for patients with single ventricle circulation. Fenestration Fontan has an advantage for maintaining cardiac output and reducing central venous pressure in the early stage after surgeries. However, its effect on hemodynamics in the long term is still unclear. It is also difficult to evaluate the simple effect of fenestration on hemodynamics in vivo since it is usually applied to the patients with deteriorated circulation.
Methods and results: We constructed a computational model for Fontan circulation. Effects of change in the cardiovascular parameters on Fontan hemodynamics were compared with and without the fenestration. When the baseline ventricular contractility (Ees) was enhanced from 1mmHg/ml to 3mmHg/ml, cardiac index (CI), O2 delivery and central venous pressure (CaVP) improved further with fenestration. Deteriorated baseline diastolic function assessed by ventricular relaxation (τ) and diastolic stiffness (B) reduced the beneficial effect of fenestration on CI, CVP and oxygen delivery. Although fenestration ameliorated CVP rise and CI reduction in patients with high pulmonary vascular resistance (Rp), SaO2 was markedly reduced despite preserved systemic O2 delivery. The simulation also shows that fenestration works well within the heart rates (HR) ranges from about 75beats/min (bpm) to 100bpm.
Conclusions: Fenestration improves Fontan hemodynamics. The effects of fenestration vary depending upon the baseline cardiovascular functions. Although fenestration is usually applied in patients with suboptimal cardiovascular functions in clinical situation, this study shows that it works best in the patients with good cardiac function and low Rp. Also, future studies in cohorts of fenestrated Fontan patients with good baseline cardiovascular functions may provide insights on beneficial long-term effects of fenestration.
Abstract
Background: Fontan procedure is a palliative surgery for patients with single ventricle circulation. Fenestration Fontan has an advantage for maintaining cardiac output and reducing central venous pressure in the early stage after surgeries. However, its effect on hemodynamics in the long term is still unclear. It is also difficult to evaluate the simple effect of fenestration on hemodynamics in vivo since it is usually applied to the patients with deteriorated circulation.
Methods and results: We constructed a computational model for Fontan circulation. Effects of change in the cardiovascular parameters on Fontan hemodynamics were compared with and without the fenestration. When the baseline ventricular contractility (Ees) was enhanced from 1mmHg/ml to 3mmHg/ml, cardiac index (CI), O2 delivery and central venous pressure (CaVP) improved further with fenestration. Deteriorated baseline diastolic function assessed by ventricular relaxation (τ) and diastolic stiffness (B) reduced the beneficial effect of fenestration on CI, CVP and oxygen delivery. Although fenestration ameliorated CVP rise and CI reduction in patients with high pulmonary vascular resistance (Rp), SaO2 was markedly reduced despite preserved systemic O2 delivery. The simulation also shows that fenestration works well within the heart rates (HR) ranges from about 75beats/min (bpm) to 100bpm.
Conclusions: Fenestration improves Fontan hemodynamics. The effects of fenestration vary depending upon the baseline cardiovascular functions. Although fenestration is usually applied in patients with suboptimal cardiovascular functions in clinical situation, this study shows that it works best in the patients with good cardiac function and low Rp. Also, future studies in cohorts of fenestrated Fontan patients with good baseline cardiovascular functions may provide insights on beneficial long-term effects of fenestration.
Introduction
The Fontan procedure has been developed as a comprehensive palliative surgery for patients with single ventricular circulation, and has led to significant improvement in patient prognosis. However, since the procedure involves bypassing the right side of the heart, high central venous pressure (CVP) is required for maintaining optimal ventricular preload and subsequent cardiac output [1]. As a result, patients often suffer from postoperative complications associated with venous congestion, such as ascites and pleural effusion, which contribute to early mortality post Fontan procedure. A fenestrated Fontan procedure helps to maintain cardiac output and limit the CVP elevation by allowing right-to-left shunting through the fenestration. This has been reported to reduce morbidity and early mortality after the surgery [2,3].
However, it remains unclear whether the fenestration in this circulation provides long-term benefits. Previous studies on the long term effects of fenestration are conflicting, [4- 7] with some studies emphasizing the positive impact of terminating the fenestrations, as represented by higher blood oxygen saturation and better exercise capacity [8]. However, diverse cardiovascular phenotypes in Fontan patients, coupled with underlying systemic complications, have been a roadblock in proper patient matching in studies conducted so far. Further, this has precluded direct analyses for distinguishing the beneficial effects of fenestration in a chronic setting from the adverse ones.
In this context, computational (simulation) studies represent a lucrative alternative approach as they enables an unbiased and robust quantification of the cardiac indices observed as a result of changes in a single hemodynamic component.9 Therefore, we sought to comprehensively examine the effect of fenestration on Fontan hemodynamics using a well-established mathematical model of the cardiovascular system [9, 10]. We hypothesized that the effects of fenestration are not uniform, and vary depending on baseline cardiovascular functions. Therefore, long-term effects of fenestration should be evaluated on the basis of comparisons between patients characterized by similar baseline cardiovascular functions.
Methods
Modeling
We constructed a computational model for the fenestrated Fontan circulation, which examined how baseline cardiovascular functions affect fenestrated Fontan hemodynamics. The model is a lumped parameter model based on the 3-element Windkessel model and coupled with the time-varying elastance model the atrium and ventricle as previously reported [10-12]. Briefly, both systemic and pulmonary circulations were modeled based on modified 3-element Windkessel impedances using capacitances, proximal characteristic resistances, and peripheral resistances [13,14]. Atrial and ventricular chambers were modeled according to the modified time-varying elastance model. End-diastolic pressure-volume relationship (EDPVR) and end-systolic pressure-volume relationship (ESPVR) as ventricular diastolic and contractile properties, respectively, were defined as follows:
where Ped and Ved represent end-diastolic pressure and end-diastolic volume, respectively, A and B are constants, and Vo is the volume at which end-systolic pressure (Pes) assumes a value of 0 mm Hg. [15] B is a stiffness constant that represents ventricular diastolic stiffness independent of loading conditions [16]. The Pes and volume (Ves) in the ESPVR are interrelated as:
where Ees is the maximal elastance that represents a loadindependent measure of ventricular contractility. Ventricular function was modeled based on the modified time-varying elastance model that was fairly uniform and independent of loading conditions, contractile state, and heart rate [17-20]. The normalized elastance curve was approximated in the present study as the following equations [21]:
where Tmax and τ represent the time taken to achieve maximal elastance and the relaxation time constant, respectively [22]. Tmax was assumed to be proportional to the QT interval, which was in turn, expressed as a function of the heart rate [23].
For atrial chambers, a simpler time-varying elastance model, given by the following equations, was used:
where Emax and Emin are the maximal and minimal atrial elastances [24].
Inlet and outlet valves were represented as diodes that permitted unidirectional flow of the blood. Flow through the valves was assumed to follow Bernoulli’s principle. The contribution of blood inertia to the flow was taken into account as follows:
where Aavv is the area of the time-varying atrioventricular valve and Lavv is the coefficient of inertial terms [25]. The right ventricle (RV) was excluded from the circuit used for modeling the Fontan circulation, and a fenestration was created between the Fontan route and the left atrium with appropriate resistance. The baseline values of each parameter are listed in Table 1 [18, 26]. Effective arterial elastance was calculated as the value of Pes divided by the stroke volume (SV) [27]. Capacity of O2 delivery was calculated as arterial oxygen saturation (SaO2) multiplied by cardiac index (CI). The model was constructed using MATLAB and Simscape (MathWorks, Inc.). For this, differential algebraic equations based on implicit numerical differentiation formulae were solved.
Table 1: Baseline Values of Each Parameter
| Ventricular parameters | SV |
|---|---|
| End-systolic elastance (Ees), mm Hg/mL Unstressed volume (Vo) Time to end-systole (Tes), ms Relaxation time constant (τ), ms Scaling factor for EDPVR (A), mm Hg Exponent for EDPVR (B), mmHg/mL |
1.6 0 340 35 0.35 0.03 |
| Atrial parameters | SA |
| Maximal elastance (Ees), mm Hg/mL Minimal elastance (Emin), mm Hg Unstressed volume (Vo) Time to maximal elastance (Tmax), ms |
0.2 0.12 0 120 |
| Circulation parameters | Systemic |
| Systemic vascular resistance (Rs), RUm2 Pulmonary vascular resistance (Rp), RUm2 Arterial resistance (Rs), dyn·s·cm−5 Characteristic resistance (Rc), dyn·s·cm−5 Venous resistance (Rv), dyn·s·cm−5 Arterial capacitance (Cs), mL/mm Hg Venous capacitance, mL/mm Hg |
15 2 1280 56 20 1.32 70 |
| Atrioventricular valve | Mitral |
| Effective orifice area, cm2 Coefficient for the inertial term, mm Hg·s2·mL−1 |
5 0.0002 |
| Other parameters | |
| Heart rates, beats/min PR duration, ms Total blood volume, mL Total stressed volume (Vs), mL Total unstressed volume (Vu), m |
75 120 5500 640 4960 |
EDPVR, end-diastolic pressure-volume relationship; SA, single atrium; SV, single ventricle
Protocols
The effect of changes in cardiovascular parameters on Fontan hemodynamics in the presence of fenestration was compared to that without fenestration in the Fontan circuit. The parameters selected for the comparative analysis, known to be key cardiovascular properties that affect Fontan hemodynamics, [28] were as follows:
- 1) ventricular contractility assessed by Ees,
- 2) ventricular diastolic function assessed by relaxation time constant (τ) and ventricular diastolic stiffness (B),
- 3) Pulmonary vascular resistance (Rp), and
- 4) Heart rates (HR)
Results
Baseline cardiovascular parameters (as listed in Table 1) yielded Fontan circulation and the results are summarized in Table 2. The hemodynamic variables were similar to those observed in a clinical setting of Fontan circulation, thereby confirming the validity of this model. Creation of the fenestration resulted in an 11 % increase in CI, along with a slight reduction in CVP at the expense of lower values of SaO2 (Table 2).
Table 2: Hemodynamics of Fontan Circulation According To Baseline Parameters
| Parameters | Fenestration (-) | Fenestration (+) |
|---|---|---|
| Cardiac index, L/min/m2 Pulmonary blood flow index (QpI), L/min/m2 Aortic pressure (systolic/diastolic/mean), mmHg Central venous pressure (CVP), mmHg Arterial oxygen saturation (SaO2), % Mixed venous oxygen saturation (SvO2), % |
3.1 3.1 80/45/62 12 99 60 |
3.5 2.3 88/52/67 11.4 88 65 |
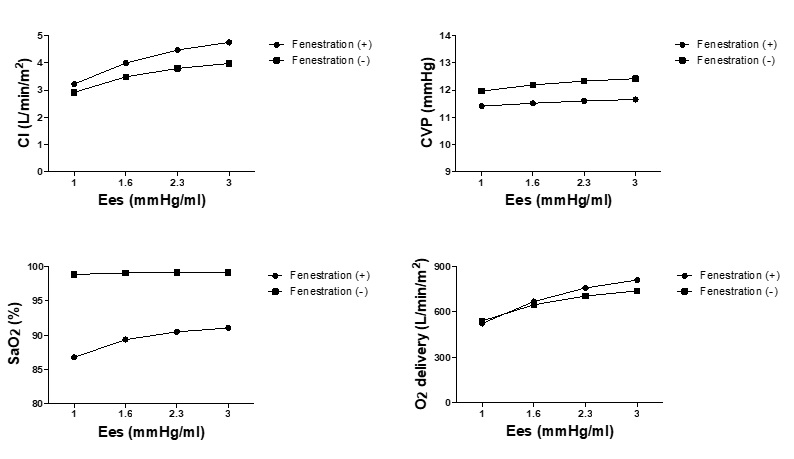
Figure 1 shows the effect of ventricular contractility on Fontan hemodynamics with and without fenestration. Baseline Ees was varied from 1 to 3 mmHg/ml, while other cardiovascular parameters remained the same. The difference between the two curves for CI, O2 delivery, and CVP becomes larger when the baseline values of Ees is increased, indicating that better baseline contractile function leads to greater beneficial effects of fenestration in terms of increasing CI and O2 delivery and reducing CVP; SaO2 could be maintained at a level more than 90 %. Notably, fenestrations created on patients with poor contractility further worsened the oxygen delivery, as indicated by the lower O2 delivery values with fenestration compared to that without, at the Ees of 1 mmHg/ml.
Figure 1: Changes in hemodynamics in response to changes in ventricular contractility (maximal elastance). Cardiac index, central venous pressure, SaO2 and O2 delivery in response to the change of the maximal elastance (Ees) in the models of Fontan circulation with and without fenestration are shown. Ees represents the maximal elastance; CI, cardiac index; CVP, central venous pressure.
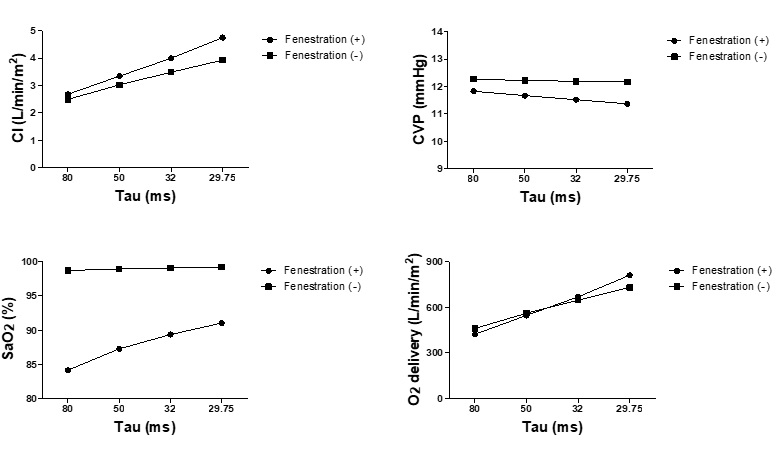
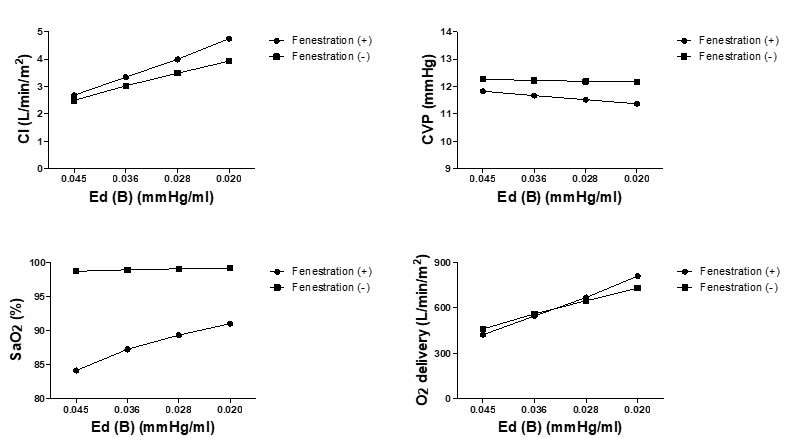
The influence of baseline diastolic function assessed by ventricular relaxation (τ) and diastolic stiffness (B), on fenestrated Fontan circulation is evident from Figures 2 and Figure 3, respectively. Similar to the effect of contractility (Ees), diastolic dysfunction represented by delayed relaxation and increased ventricular diastolic stiffness reduced the beneficial effects of fenestration on CI, CVP, and oxygen delivery. In addition, fenestration created on patients with severely delayed relaxation times (τ = 80 ms) and increased stiffness (B = 0.045 mmHg) further worsened the systemic oxygen delivery.
Figure 2: Changes in hemodynamics in response to changes in ventricular relaxation. Cardiac index, central venous pressure, SaO2 and O2delivery in response to the change of the relaxation time constant (τ) in the models of Fontan circulation with and without fenestration are shown. Τau represents the relaxation time constant; CI, cardiac index; CVP, central venous pressure.
Figure 3: Changes in hemodynamics in response to changes in ventricular diastolic stiffness. Cardiac index, central venous pressure, SaO2 and O2 delivery in response to the change of ventricular diastolic stiffness (B) in the models of Fontan circulation with and without fenestration are shown. Ed (B) represents ventricular diastolic stiffness; CI, cardiac index; CVP, central venous pressure.
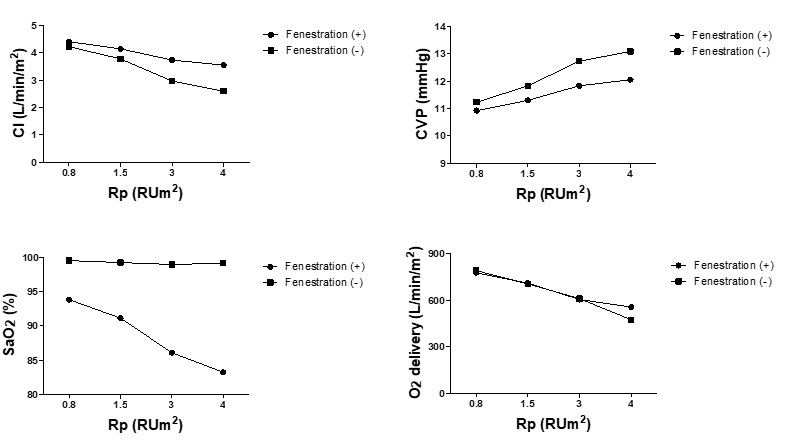
Figure 4 shows the effects of changes in Rp on fenestrated Fontan hemodynamics. Contrary to the contribution of cardiac functions, patients with lower Rp were found to benefit less from fenestration, although it still improved CI, O2 delivery, and CVP. In contrast, fenestration ameliorates the increase in CVP and decrease in CI at the expense of markedly reduced SaO2 in patients with high Rp despite preserved systemic O2 delivery. Of note, when Rp becomes higher than 3 Rum [2], SaO2 decreases to the levels observed in Glenn circulation (i.e less than 85 %). Therefore, values of Rp corresponded to improved CI and CVP values, whereas SaO2 was preserved Sas a right heart bypass circulation in fenestrations limited to relatively small ranges (less than 3 Rum2) (Figure 4).
Figure 4: Changes in hemodynamics in response to changes in pulmonary vascular resistance. Cardiac index, central venous pressure, SaO2 and O2 delivery in response to the change of pulmonary vascular resistance (Rp) in the models of Fontan circulation with and without fenestration are shown. Rp represents pulmonary vascular resistance; CI Cardiac index; CVP Central venous pressure.
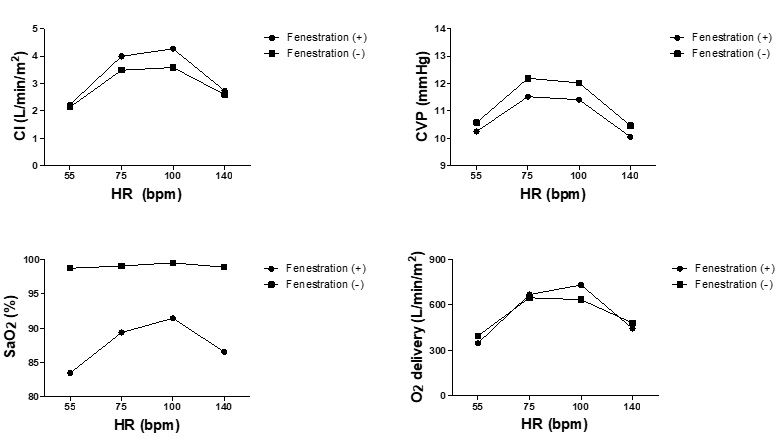
Effects of HR on fenestration are shown in Figure 5. Fenestration works well within HR ranges of about 75 to 100 beats/min (bpm), whereas the beneficial effects of fenestration decrease when HR is too low or too high.
Figure 5: Changes in hemodynamics in response to changes in heart rates. Cardiac index, central venous pressure, SaO2 and O2 delivery in response to the change of heart rates (HR) in the models of Fontan circulation with and without fenestration are shown. HR represents heart rates; CI Cardiac index; CVP Central venous pressure.
Discussion
In the present study, we investigated the effects of fenestration on Fontan hemodynamics with respect to changes in cardiovascular functions. We report several novel findings in the present study. Fenestration considerably improved the Fontan hemodynamics. However, the beneficial effects of fenestration were not uniform, and depended strictly upon baseline cardiovascular functions, possibly leading to conditions where fenestration could worsen Fontan hemodynamics.
As shown in Figures 1, 2, and 3, fenestration improved CI and oxygen delivery to a greater extent in patients with better ventricular contractile and diastolic functions. This could be attributed to an increase in CI that enhanced oxygen delivery despite the reduction of SaO2 caused by right-toleft shunting through the fenestration. Our earlier clinical report on fenestrated Fontan circulation supports this result [6]. We showed that an increase in CI with a dobutamine infusion correlated well with the increase in fenestration flow, and the latter increased with increased mixed venous saturation. This indicated that more oxygenated blood through the fenestration contributes to improved CI, helps to mitigate a decrease in SaO2, and works synergistically to increase oxygen delivery. It is also important to note that changes in O2 delivery and CI according to ventricular functions shown in Figures 1 to 3 occur when changes in CVP were within physiologically acceptable range for the Fontan circulation (i.e., less than 13 mmHg). Although CVP is an important parameter for creating fenestration, the fact that the influence of fenestrations vary even within the same CVP levels need to be taken into consideration when deciding the indication of fenestration creation in the clinical settings.
Changes in the effect of fenestration on Fontan hemodynamics, in terms of changes in Rp and HR, deserve further discussion. Patients with increased Rp have been considered as good candidates for the fenestrated Fontan procedure [29,30]. Our results confirmed that patients with lower Rp benefited less from fenestration. However, fenestration in patients with Rp higher than 3 RU m2 resulted in the marked reduction of SaO2. These results were obtained with other cardiovascular functions set within the normal range. Therefore, in patients with impaired ventricular function combined with increased Rp (where fenestrated Fontan surgery is often suggested in the clinical setting), the upper limit of Rp value that can improve hemodynamics without compromising SaO2 should be lower than 3 RU m2. This is very important notion, because fenestrated Fontan patients with high Rp may not benefit from Fontan circulation compared to staying in Glenn circulation, and thus should be considered when checking the indication of fenestration creation in Fontan surgery. In addition to the influence of changes in Rp values, changes in HR affect the fenestration effects in various ways. As clearly demonstrated in Figure 5, fenestration improves hemodynamics more effectively within the HR ranges of about 75 to 100 bpm. The beneficial effects of fenestration reduce when the values of HR lie outside these ranges, suggesting the importance of HR modulation when managing patients with fenestrated Fontan circulation. These results were also consistent with our previous clinical study demonstrating that rapid atrial pacing compromised hemodynamic advantages of fenestration [6].
Therefore, although the fenestrated Fontan procedure has generally been applied to high risk patients with elevated Rp and/or impaired cardiac functions, we should acknowledge that fenestration works best in Fontan patients with good cardiac function and low Rp. Further, a fenestrated Fontan procedure performed on patients with marked cardiac dysfunction or high Rp reduces systemic oxygen delivery or causes a significant reduction in SaO2 with a minimal improvement of cardiac output. In addition, since the beneficial effects of fenestration were observed within the limited range of HR, HR control by pacemaker, or medications, and/or life style modification would be beneficial for maximizing the benefits of fenestration.
In conclusion, the effect of fenestration depends upon baseline cardiovascular functions. Therefore, future studies in cohorts of fenestrated Fontan patients with good baseline cardiovascular functions may provide insights on beneficial long-term effects of fenestration. We have to define the indication of fenestration for patients with high Rp and cardiac dysfunction, such that they can gain the benefit of Fontan circulation compared to staying in Glenn circulation.
There are no references