Journal Name: Journal of Cardiovascular Disease and Medicine
Article Type: Case Report
Received date: 23 June, 2018 Accepted date: 30 June, 2018 Published date: 06 June, 2018
Citation: Habash F, Shanbhag A, Card P, Gheith Z, Guthrie J, et al (2018) Recurrent Contact Dermatitis Secondary to Topical Skin Adhesive after Cardiac Implantable Electronic Device Procedure. J Cardiovas Disea Medic 1:1 (53-55).
Copyright: © 2018 Habash F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Dermabond® is a topical skin adhesive that is used as an alternative to sutures, thus making wound closure simple and efficient without the need of sterilized equipment, anesthesia or removal procedures. Such topical adhesive is used frequently to help close small wounds after device implantations or upgrades as it is easy to apply with no pain. Though rare, Dermabond® is capable of causing an inflammatory reaction as its main ingredient (cyanoacrylate) degenerates in the skin to release formaldehyde. We herein report 2 cases of skin reaction around device site that was thought to be infectious but turned out to be Dermabond induced contact dermatitis.
Keywords
Dermabond, contact dermatitis, wound dehiscence, wound healing, device complications.
Abstract
Dermabond® is a topical skin adhesive that is used as an alternative to sutures, thus making wound closure simple and efficient without the need of sterilized equipment, anesthesia or removal procedures. Such topical adhesive is used frequently to help close small wounds after device implantations or upgrades as it is easy to apply with no pain. Though rare, Dermabond® is capable of causing an inflammatory reaction as its main ingredient (cyanoacrylate) degenerates in the skin to release formaldehyde. We herein report 2 cases of skin reaction around device site that was thought to be infectious but turned out to be Dermabond induced contact dermatitis.
Keywords
Dermabond, contact dermatitis, wound dehiscence, wound healing, device complications.
Background
Dermabond® is a topical skin adhesive that is used as an alternative to sutures, thus making wound closure simple and efficient without the need of sterilized equipment, anesthesia or removal procedures [1]. Dermabond® contains 2-octyl-cyanoacrylate, which rapidly polymerizes upon contact with keratin to achieve its adhesive capability. A possible reason for the low number of reported sensitization cases from Dermabond® is that antigen-presenting cells (APCs) are capable of attaching only to monomers and not polymers [1,2]. Adhesive tape, on the other hand, is notorious for causing contact dermatitis. Though rare, Dermabond® is capable of causing an inflammatory reaction as cyanoacrylate degenerates in the skin to release formaldehyde [3]. We herein report 2 cases of Dermabond induced contact dermatitis.
Case Report
Case 1
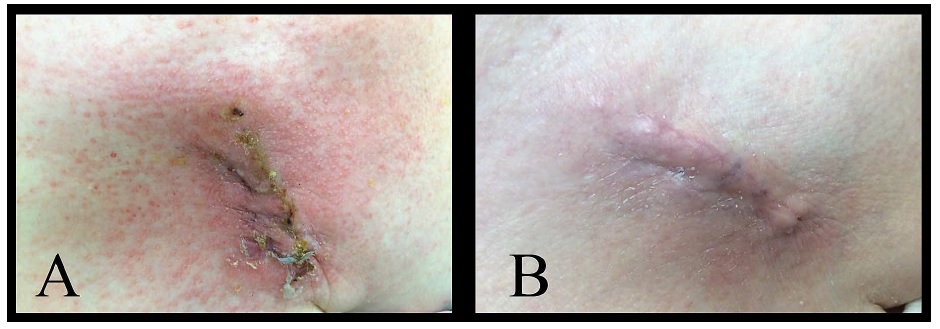
An 81-year-old female with dual-chamber pacemaker placed five years ago required a generator change. During her post-generator change wound check clinic visit, she reported itching and redness around the incision and it soon developed scabs with serous discharge (Figure 1A). Due to risk of device infection, the patient was hospitalized and started on antibiotics after wound and blood cultures were collected. Dermatology was consulted and felt it was contact dermatitis secondary to Dermabond® use after suturing. The scabs and crusting were likely from wound healing with a low likelihood of infection. Cultures did not exhibit any growth and the rash improved with the application of triamcinolone ointment on the dermatitis area and Vaseline® (petroleum jelly) on the incision. On repeat application of Dermabond®, recurrence of contact dermatitis was noted indicating Dermabond® as the probable cause of skin reaction. Dermabond® residuals were scraped off and steroid cream and Vaseline® were continued and led to complete resolution of the dermatitis. The end result was a well-healed incision site (Figure 1B).
Figure 1: A) Contact dermatitis with crusts and skin erythema. B) Resolution of contact dermatitis.
Case 2
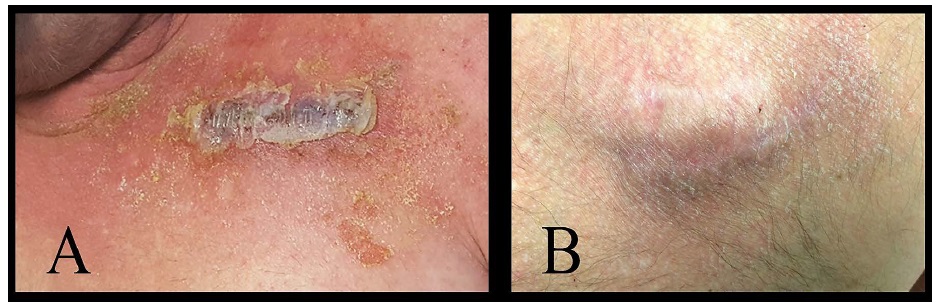
A 47-year-old man with dual-chamber ICD placed 3 years ago presented for generator change. During postgenerator change wound check clinic visit, he was noted to have increasing pain and scant discharge over the wound for which he was admitted to the hospital to rule out device infection. Wound and blood cultures were collected, and IV antibiotics were started. During the hospital stay, the wound was stapled due to wound dehiscence and Dermabond® was then applied. In the following 24 hours, he developed diffuse rash with vesicles over the wound and the area where Dermabond® was applied (Figure 2A). Dermatology evaluated the patient and diagnosed him with Dermabond® induced contact dermatitis. The decision was made to start the patient on topical hydrocortisone 2.5% around the wound in addition to Vaseline® over the wound edges. Symptoms started to improve after hydrocortisone application and his rash resolved in the following 12 hours. One week later, the wound was completely healed, and the staples were then removed (Figure 2B) [4].
Figure 2: A) Contact dermatitis with extensive skin desquamation and crusting with some vesicular changes. B) Resolution of contact dermatitis with residual minimal desquamation.
Discussion
Reported cases of Dermabond®-induced contact dermatitis in the past included those with prior sensitization to Dermabond as well as first time exposure [5]. Some attribute the reaction to the prolonged application of Dermabond® or the sheer excessive quantity of Dermabond® applied [6]. Cases include those with superficial wound closures after lipoma extraction [7], osteotomy [8], wound closure after joint replacement [9], breast lumpectomy [5], breast reduction surgery [10], abdominoplasty wound closure [1], arteriovenous fistula incision [11] and other minor procedures. For our cases, the differential diagnosis included device infection, but the absence of fever, induration and warmth over the device site made infection an unlikely cause. The presentation of itching and redness without pain is also not consistent with infection but is rather more consistent with dermatitis. In a scenario where rash is concerning for infection, adhesive should be removed to make sure that contact dermatitis or hypersensitivity is neither the cause nor a contributing factor to the rash. Even though the reaction to Dermabond® is rare, considering the currently increasing use of skin tissue adhesives, physicians should be aware of this reaction and should limit repetitive application of this agent if such reaction occurs. Management of Dermabond®-induced dermatitis includes removal of Dermabond® from over the incision site without injuring the wound edges followed by application of topical steroids for a short period of time [1]. It is also imperative to rule out infection (particularly in patients with foreign bodies and devices). Other products, such as Vaseline®, were helpful in our cases and can be used to provide an occlusive layer and decrease the risk of infection.
Conclusion
Dermabond®-induced contact dermatitis is an uncommon reaction and should be recognized by physicians who use such agents for wound management. Ruling out infection is essential, but when contact dermatitis is suspected, like in our patients, use of topical steroids and removal of adhesive will provide effective and rapid resolution of skin reaction. As stated above, Vaseline® is another agent that is very useful to combine with topical steroids when caring for these wounds.
Acknowledgements and Conflict of Interest
Authors have no conflicts of interest to declare. Parts of this manuscript were presented as an oral presentation at the World Congress of Cardiology 2017 in Vancouver, Canada.
Funding Source
This manuscript has not been funded by any source.
Hivnor CM and Hudkins ML (2008) Allergic Contact Dermatitis After Postsurgical Repair With 2-Octylcyanoacrylate. Arch Dermatol 144: 814-815. [ Ref ]
Tomb RR, Lepoittevin JP, Durepaire F, Grosshans E (1993) Ectopic contact dermatitis from ethyl cyanoacrylate instant adhesives. Contact Dermatitis 28: 206-208. [ Ref ]
Malten KE (1979) Recently reported causes of contact dermatitis due to synthetic resins and hardeners. Contact Dermatitis 5: 11-23. [ Ref ]
Habash F, Paydak O, Pothineni NV, Card P, Sewani A (2018) Stapling for Wound Dehiscence After Cardiac Implantable Electronic Device Implantation. Arch Turkish Soc Cardiol 46: 242-247. [ Ref ]
Caton AM and Dauphine C (2014) Allergic contact dermatitis after repeated exposure to dermabondTM. Am Surg 80: 520-522. [ Ref ]
Perry AW and Sosin M (2009) Severe Allergic Reaction to Dermabond. Aesthetic Surg J 29: 314-316. [ Ref ]
Sachse MM, Junghans T, Rose C, Wagner G (2013) Allergic contact dermatitis caused by topical 2-octyl-cyanoacrylate. Contact Dermatitis 68: 317-319. [ Ref ]
Sato M, Inomata N, Aihara M (2017) A case of contact dermatitis syndrome caused by Dermabond®, followed by contact dermatitis caused by false eyelash glue and Aron Alpha® glue: possibility of crossreactions among cyanoacrylates. Contact Dermatitis 77: 414-415. [ Ref ]
El-Dars LD, Chaudhury W, Hughes TM, Stone NM (2010) Allergic contact dermatitis to Dermabond® after orthopaedic joint replacement. Contact Dermatitis 62: 315-317. [ Ref ]
Howard BK and Downey SE (2010) Contact Dermatitis from Dermabond. Plast Reconstr Surg 125: 252e-253e. [ Ref ]
Bitterman A and Sandhu K (2017) Allergic contact dermatitis to 2-octyl cyanoacrylate after surgical repair: Humidity as a potential factor. JAAD Case Reports 3: 480-481. [ Ref ]