Journal Name: Journal of Clinical Case Reports and Trials
Article Type: Research
Received date: 03 June, 2018
Accepted date: 01 August, 2018
Published date: 09 August, 2018
Citation: Daniel L, Rarichan L, Jose M, Binoy MS, Rajalingam B (2018) An Investigation on Drug Related Problems in Pediatrics of a Tertiary Care, Private, Teaching Hospital at Coimbatore. J Clin Case Rep Trials. Vol: 1, Issu: 2 (01-07).
Copyright: © 2018 Daniel L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Aim: Drug Related Problem is defined as an event or circumstances involving drug therapy. The aim of this study was to identify & assess the drug related problems in the pediatric population and to classify the identified DRPs based on the PCNE classification and then to recommend prevention strategy for the identified DRPs in pediatrics.
Materials and Methods: The study was a prospective observational study to identify and to classify the identified DRPs based on the PCNE classification and to recommend prevention strategy for the identified DRPs in pediatric patients of either sex below the age group of 12 years during the study period between February to August 2016 and a separate data entry form for incorporating patient details were designed. The obtained results were analyzed using the chi-square test.
Results: A total of 763 drugs were prescribed for 150 patients during the study period. 425 drug related problems were observed in the study population. The length of stay of the study population was analyzed and the average number of days of hospital stay was found to be 4.52 ± 2.40 days. The average number of injections per patient was found to be 2.84 ± 1.91. The prescriptions were analyzed for drugdrug interactions and it was found that 72% prescriptions had drugdrug interactions. The average number of drug-drug interactions was found to be 0.74 ± 4.38. The study population was evaluated for ADRs and found to have 08 ADRs. The results revealed that 50% of ADRs reported were probable and the remaining were possible. Prescriptions were analyzed, and the reports revealed a total of 44 over dosages and a total of 13 under dosages. The prescriptions were analyzed for Contraindicated combinations and a total of 22 such events were found. The analysis of prescriptions revealed about 83 incidences where no drug was prescribed for an indication. The identified DRPs were classified based on PCNE classification system (Version 6.2) into problem, causes, interventions and outcome of intervention and these were further categorized into primary and secondary domain.
Conclusion: The study proved to be a convincing evidence for the role of clinical pharmacist and in providing pharmaceutical care program which improves patient outcomes.
Abstract
Aim: Drug Related Problem is defined as an event or circumstances involving drug therapy. The aim of this study was to identify & assess the drug related problems in the pediatric population and to classify the identified DRPs based on the PCNE classification and then to recommend prevention strategy for the identified DRPs in pediatrics.
Materials and Methods: The study was a prospective observational study to identify and to classify the identified DRPs based on the PCNE classification and to recommend prevention strategy for the identified DRPs in pediatric patients of either sex below the age group of 12 years during the study period between February to August 2016 and a separate data entry form for incorporating patient details were designed. The obtained results were analyzed using the chi-square test.
Results: A total of 763 drugs were prescribed for 150 patients during the study period. 425 drug related problems were observed in the study population. The length of stay of the study population was analyzed and the average number of days of hospital stay was found to be 4.52 ± 2.40 days. The average number of injections per patient was found to be 2.84 ± 1.91. The prescriptions were analyzed for drugdrug interactions and it was found that 72% prescriptions had drugdrug interactions. The average number of drug-drug interactions was found to be 0.74 ± 4.38. The study population was evaluated for ADRs and found to have 08 ADRs. The results revealed that 50% of ADRs reported were probable and the remaining were possible. Prescriptions were analyzed, and the reports revealed a total of 44 over dosages and a total of 13 under dosages. The prescriptions were analyzed for Contraindicated combinations and a total of 22 such events were found. The analysis of prescriptions revealed about 83 incidences where no drug was prescribed for an indication. The identified DRPs were classified based on PCNE classification system (Version 6.2) into problem, causes, interventions and outcome of intervention and these were further categorized into primary and secondary domain.
Conclusion: The study proved to be a convincing evidence for the role of clinical pharmacist and in providing pharmaceutical care program which improves patient outcomes.
Introduction
The term pediatrics is derived from the Greek word ‘pedio-pais, ‘paidos’ meaning a child or donating relationship to a child (pedio), ‘iatrike’ meaning surgery or medicine and ‘ics’, suffix of a subject of science. It has come to mean the science of child care in the present day and includes planned prevention and curative care of children [1]. Infancy and childhood are rapid growth and development. The various organs, body systems and enzymes that handle drugs developed at different rates; hence, drug dosage, formulation, response to drugs and adverse reactions vary throughout childhood [2]. Clinician’s needs to ensure not only that toxicity is kept to a minimum but also that children are not denied the use of appropriate medicines. Drug use in children may be accompanied by problems not seen in adults or cause adverse drug reactions that are more frequent than in adults [3]. The rate of absorption was correlated with age, being much slower in neonates than in older infants and children. Changes in the absorption rate would appear to be of minor importance when compared to the agerelated differences of drug distribution and excretion of medications. The percentage of total body weight, the total body water and extracellular fluid volume decrease with age. Despite normal blood pH, free fatty acid and bilirubin levels in infants, binding to plasma proteins is reduced as a result of low concentrations of both globulins and albumin. It has been suggested that binding values comparable with those seen in adults are reached within the third year of life for acidic drugs, whereas for basic drugs adult values are not reached until the age of 7 to 12 years. At birth the majority of the enzyme systems responsible for drug metabolism are either absent or present in considerably reduced amounts compared with adult values, and evidence indicates that the various systems do not mature at the same time. This reduced capacity for metabolic degradation at birth is followed by a dramatic increase in the metabolic rate in the older infants and young child. The anatomical and functional immaturity of the kidneys at birth limits renal excretory capacity. Below 3–6 months of age the glomerular filtration rate is lower than that of adults but may be partially compensated for by a relatively greater reduction in tubular reabsorption. Tubular function matures later than the filtration process [2].
Drug-related problem (DRP) is defined as ‘an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes’ [4]. Addressing DRPs has become a priority, owing to the complexity of today’s drug therapy, which consequently makes appropriate drug prescribing increasingly challenging [5].
Here we adopt the most suitable and highly recognized system of classification - Pharmaceutical Care Network Europe (PCNE) system (version 4.0) (PCNE). The original classification was created in 1999 by pharmacy practice researchers during a working conference of the PCNE in an effort to develop a standardized classification system that is suitable and comparable for international studies. This hierarchical system comprises separate codes for problems, causes, and interventions and is hierarchically structured. As per PCNE classification system, a DRP is an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes [4].
Methodology
Literature search
Literature survey was done in order to collect the supporting evidence for the study proposed and the literatures were gathered from various sources such as American Academy of Pediatrics, British Medical Journal, Canadian Pediatric Society, Journal of Clinical Pharmacy and Therapeutics. Databases including Micromedex, Medscape, Pub med, Science direct were also been widely used.
Study design and population
This study was a prospective and observational study conducted for a period of 6 months. A separate data entry form for incorporating patient demographic details, laboratory investigations diagnosis and drug chart (name of the medication, route & strength, dose, frequency and days of treatment) were designed. Data’s were obtained from 150 patients’ medical charts.
Inclusion criteria
Pediatric patients of both sex below the age group of 12 years with sufficient data and who were willing to participate in the study were included.
Exclusion criteria
Patients in the age of above 12 years, with insufficient data and who are not willing to participate were excluded from the study.
Methods
All the patients who met the inclusion criteria were selected and the data were collected during regular ward round participation. Patient or caregivers were informed about the study and their written consent was obtained using appropriate forms. Data entry form was used to obtain information on demographics of patient (e.g. patient name, age, gender, height, weight, date of admission and date of discharge), presenting complaints, laboratory test reports, provisional/confirmed diagnosis, drug therapy given (with brand names and generic names of each drug, dose, duration and route of therapy).
Data analysis
The data obtained was analyzed for obtaining the prevailing DRPs as per the PCNE version 6.2. The identified DRPs were classified based on PCNE classification system into problems, causes, intervention and outcome of interventions.
Results
The proposed work entitled “An investigation on drug related problems in pediatrics of a tertiary care, private teaching hospital at Coimbatore” was a prospective observational study, carried out in a 750 bedded hospital. Data were collected by using the specially designed data entry format during the regular ward rounds. The data collected were analyzed and the following results were obtained.
Study Population: A total of 624 patients got admitted to the study site during the study period. Out of which, 150 patients who were matching our inclusion criteria “pediatric patients of either sex below the age group of 12 years admitted in to the study site during the study with sufficient data and who were willing to participate in the study” were included and “patients in the age of above 12 years, with insufficient data and who were not willing to participate” were excluded from the study. The analysis of demographics had revealed that a very slight female dominant population was found, and the age categorization revealed that the study population was found more between 2-12 years of age./p>
Length of stay
Length of stay is the term to describe the duration of single episode of hospitalization. The length of stay of the study population was analyzed and the average number of days of hospital stay was found to be 4.52 ± 2.40 days. And more than two third of the patients included in the study had a minimum of 3-5 days of hospital stay.
Past medical history
The past medical history of the study population was also analyzed. Result revealed that seizure, RTI and UTI were more prevalent among the study population.
Reasons for admission
The analysis of reasons for admission to the study site had revealed that certain complaints like fever, vomiting, cough and cold, loose stools, etc. were found to be very common reasons.
Diagnosis
The provisional diagnosis of the study population was also analyzed, and the common diagnosis was found to be viral pyrexia, RTI, febrile seizures, etc.
Route of administration
Route of administration of the prescribed drugs to the study population was analyzed. The result revealed that most commonly used route of administration was injectables (50%). It was also observed that only 4.66% of the study population had not received any injections and 16.66% were given with at least one injection and rest of the population i.e., about 79% had more than one injection. The average number of injections per patient was found to be 2.84 ± 1.91.
Drugs prescribed
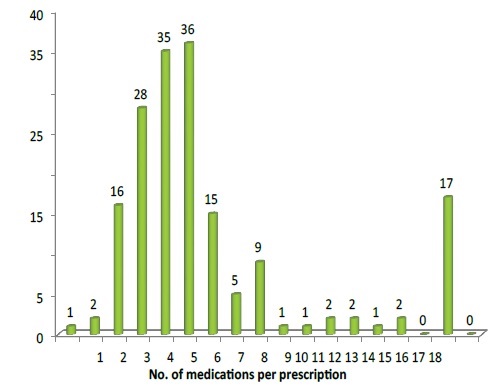
A total of 763 different drugs were prescribed to the study population. Analysis revealed that majority of the study population has received antibacterial (24.5%) and IV fluids (13.1%). It was also found that 5.85 ± 2.73 was the average number of drugs prescribed per prescription (Figure 1).
Figure 1: Number of medication per prescription.
Prevalence of DRPs
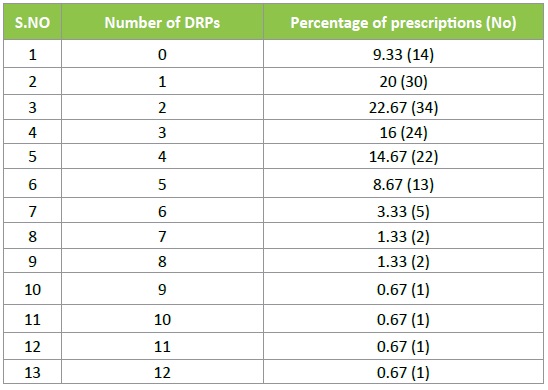
A total of 150 patients were admitted in the study site during the study period and a total of 425 DRPs were found out. Of these drug interactions were the most commonly observed DRP. The average number of DRPs per prescription was found to be 2.83 ± 2.24 (Figure 2) (Table 1).
Figure 2: Prevalence of DRP.
Table 1: No. of DRPs per prescription.
Drug interactions
The prescriptions were analyzed for drug-drug interactions and it was found that 72% prescriptions had drug-drug interactions and 28% did not have any interactions. It was also found that there were 25.41% (108) incidences of drugdrug interactions which had 59 types including 11 major, 35 moderate and 13 minor interactions. The average number of drug-drug interactions was found to be 0.74 ± 4.38.
Adverse drug reactions
The study population was evaluated for ADRs and found to have 08 ADRs and most of them were due to antibacterial and reactions included vomiting, rashes, diarrhea etc. The identified ADRs were assessed using Naranjo’s scale and then were assigned to a probability category from the total score as follows: ‘Definite’ if the overall score is 9 or greater, ‘Probable’ for score of 5-8, ‘Possible’ for 1-4 and ‘Doubtful’ if the score is 0. The results revealed that 50% of ADRs reported were probable and the remaining were possible. The results also revealed that the category of drug which was causing a greater number of ADRs was found to be antibacterial (75%) especially ceftriaxone.
Drug dosing problems
Over dose: Prescriptions were analyzed, and the reports revealed a total of 44 over dosages. The problems were addressed to the physicians with appropriate dose based on the patient’s body weight.
Under dose: Prescriptions were analyzed, and the reports revealed a total of 13 under dosages. The problems were addressed to the physicians with appropriate dose based on the patient’s body weight.
Contra indications
The prescriptions were analyzed for CI and a total of 22 such events were found.
No drug for indication
The analysis of prescriptions revealed about 83 incidences where no drug was prescribed for an indication and among that 24.10% had low levels of hemoglobin and no iron supplements were given. Other common indications for which drugs were not prescribed included diarrhea, vomiting.
Drug without indication
The prescriptions were analyzed, and the results revealed that 28.57% patients were given with Syp. Ibugesic but they were not having any complaints of pain.
DRPs classified according to the PCNE classification
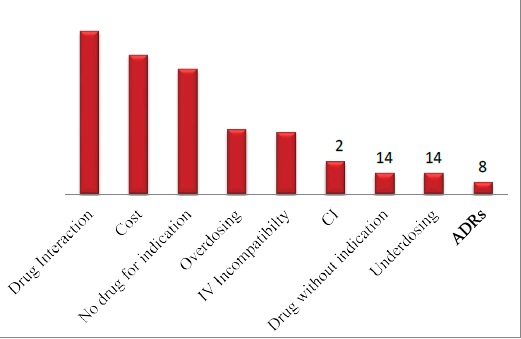
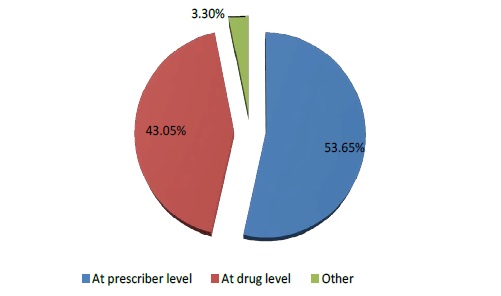
The DRPs identified were classified according to the PCNE 6.2 version classification as described earlier. Majority of the DRPs in problem domain were found under ‘others’ category followed by ‘treatment effectiveness’. The secondary domain analysis revealed that no drug for indication was found in 83 incidences. The details are given in Table 2. Analysis of causes for the existing DRPs revealed that ‘drug selection’ in primary domain and drug-drug interaction, cost comparison and no drug for indFication in secondary domain were found to be dominant. The details are given in Table 3. Interventions provided for the DRPs were categorized and the results are given in Table 4 and Figure 3. The results revealed that ‘interventions at prescriber level’ in primary domain and the ‘proposed intervention was not approved by the prescriber’ was found to be dominant. Outcome of interventions for the DRPs were categorized and the details are given in Table 4 and Figure 4. The results revealed that ‘not known’ in primary domain and ‘outcome intervention not known’ in secondary domain was found to be dominant (Table 5).
Table 2: PCNE Classification of DRPs – Problems.
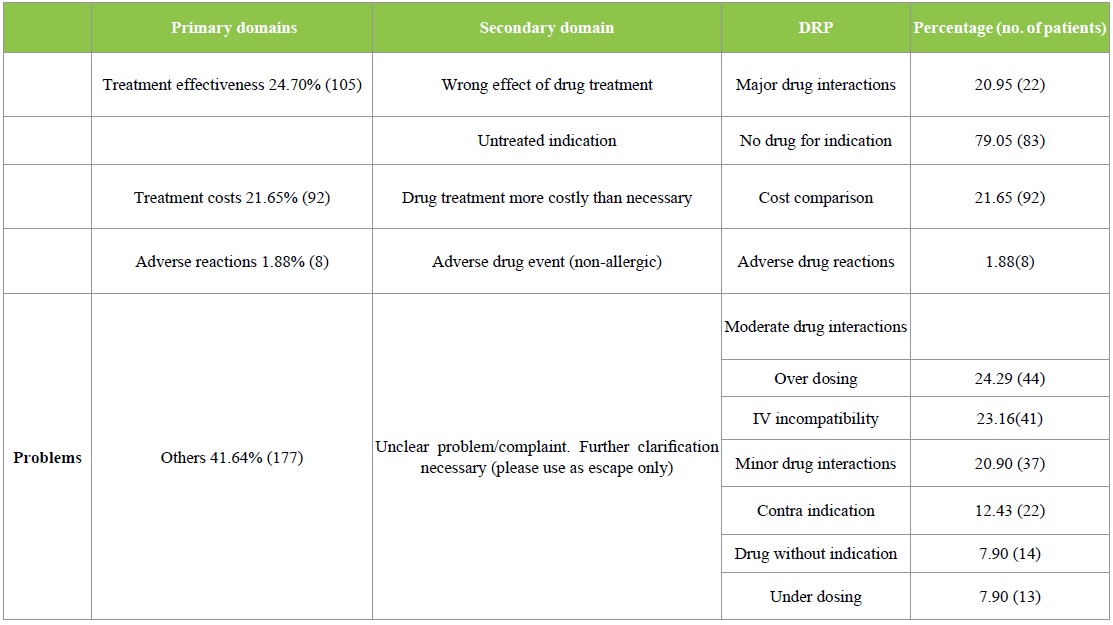
Table 3: PCNE Classification of DRPs – Causes.
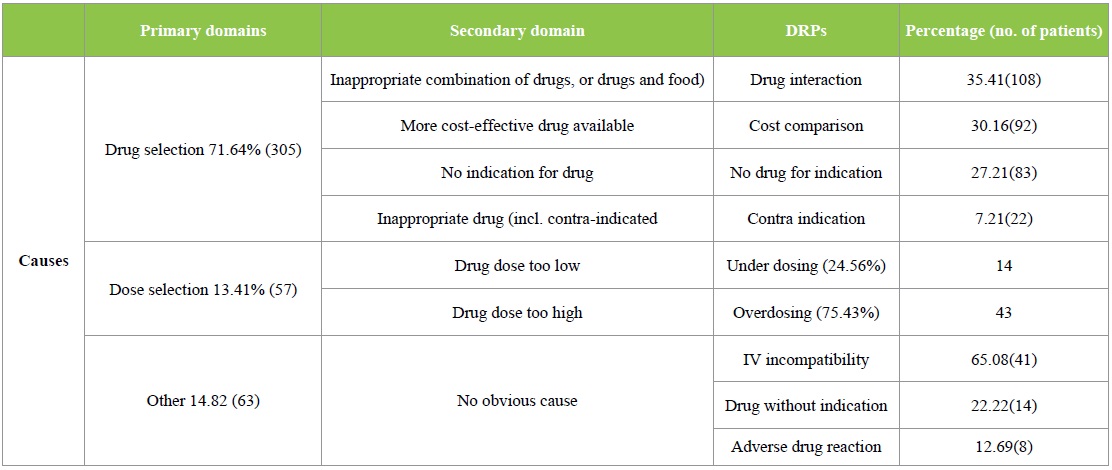
Table 4: PCNE Classification of DRPs- Interventions.
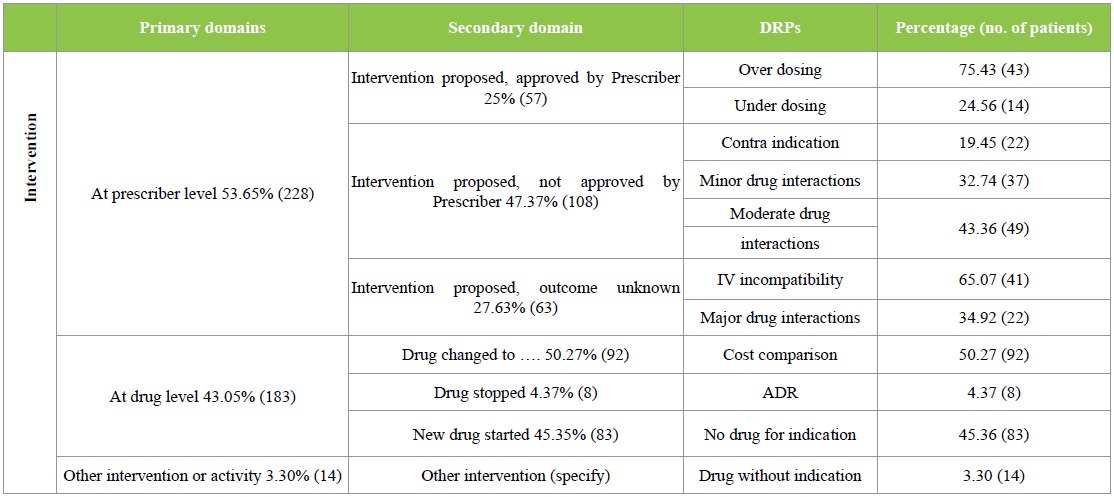
Figure 3: Interventions provided for DRPs.
Table 5: PCNE Classification of DRPs: Outcome of interventions.
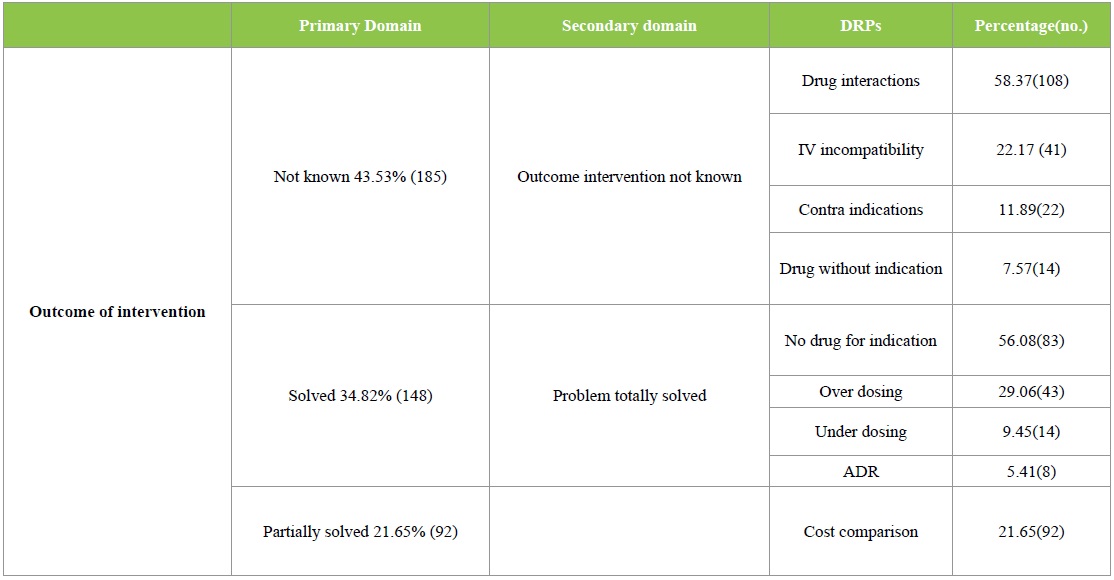
Statistical analysis
Variables like age and individual DRPs were analyzed using Chi square test and found to be statistically significant with p=0.05 and the results revealed that ADR was the only DRP which was found to be significant.
Prevention strategies
The DRPs are needed to be either prevented from reaching patients or at least the harm needs to be eliminated if it reaches the patient. The following are the prevention strategies recommended for the DRPs.
Drug-drug interactions can be prevented by avoiding concomitant use of interacting drugs, spacing the dosing intervals of drugs, discontinuing/ replacement of the drug, dosage adjustments, adopting alternative drug, use of software’s and CPOE, monitoring for early detection i.e., through close laboratory or clinical monitoring for evidence of interaction like QT interval prolongation, ECG, renal function, serum drug concentration and provide information on patient risk factors that increases the chance of adverse outcomes.
ADRs can be prevented by evaluating patient’s medical history to identify any previous incidences of ADRs, direct interview with the caregivers to identify any potential ADR and counseling patients at the time of discharge to report any adverse events occurred by using “ALERT CARDS”.
IV incompatibility can be overcome by solubilization or vigorous shaking if immiscibility occurs in case of physical incompatibility. It can be also prevented by separating the site of administration, avoid mixing of drugs at the same infusion site, changing the order of mixing, alteration of solvent, addition or substitution or omission of therapeutically inactive ingredient or completely flush out the IV set with appropriate diluents. In case of chemical incompatibility avoid administering interacting drugs together protection from light, choice of suitable pharmaceutical dosage form which reduce the possibility of oxidation (preferring solid dosage form than solution). Before the administration of drugs compatibility can be checked using available databases, eg.: Micromedex 2.0(electronic version).
Prescriptions should be carefully analyzed to make sure that drugs are prescribed for every indication and should suggest the physician to prescribe with safe and effective drug which is appropriate and approved for the particular indication by FDA/CDSCO.
Over/Under dose can be prevented by calculating dose based on patient’s body weight. Patient characteristics should be taken in consideration for safe use of drugs to avoid prescribing any contraindicated drugs.
Cost effective treatment should be practiced by prescribing drugs that cost patients less and that are available in hospital pharmacy. The hospital pharmacist has to perform drug use evaluation and recommend with cost effective alternative therapies.
Discussion
In India, about 35% of the total populations are children below 15 years of age. Majority of the childhood sickness and death are preventable by simple low-cost measures. Children always need special care to survive and thrive. Children are more prone to variety of diseases [1].
The pediatric population is a diverse and dynamic group. The use of medicines in children is an area of increasing interest. Infancy and childhood are a period of rapid growth and development. The various organs, body systems and enzymes that handle drugs developed at different rates; hence, drug dosage, formulation, response to drugs and adverse reactions vary throughout childhood. Clinician’s needs to ensure not only that toxicity is kept to a minimum but also that children are not denied the use of appropriate medicines. Drug use in children may be accompanied by problems not seen in adults or cause adverse drug reactions that are more frequent than in adults. An example of this is metoclopramide, which causes dystonia in teenagers and Parkinsonism in elderly./p>
The pediatric patient presents unique challenges throughout the medication use process as they encompass a variety of age, weight, and body surface area which require patient-specific dosing calculations. They are also more vulnerable to drug related problems than an adult, due to their differences in weight or body surface area and because of the varying ability to metabolize and excrete medications. A study conducted in Egypt on drug related problems in children with cardiac diseases concluded that over a three-month period, a total of 313 DRPs were recorded corresponding to an average of 5.22 problems per patient and the most commonly recorded problems related to drug-drug interaction (45.69%), prescribing unnecessary medication (31.95%), under-dosing (21.09%), inappropriate medication (0.96%) and adverse drug reaction (0.32%) [6]./p>
Another study was conducted at Australia to determine the incidence of hospital admissions for drug-related problems (DRPs) among children and to examine cases for causality, preventability and clinical severity. Prospective assessment involved review of case notes and parent interview to determine if an admission was associated with a DRP. The study concluded that 58 admissions (3.4%) were associated with DRPs. Noncompliance was implicated in 50%. Causality was ranked as “definite” (34.5%), “possible” (56.9%) and “doubtful” (8.6). Two-third of admissions associated with DRPs were deemed preventable [3]. Another study was conducted at London to determine drug related problems in hospitalized children and the study concluded that 147 patients suffered from 220 DRPs and the overall DRP incidence was 39.4% (95% CI 34.4 to 44.6). Incidence was highest in PICU 60.0 (95% CI 45.2 to 73.6). Dosing problems were the most frequently reported DRPs (n=76, 34.5%). 67.7% of DRP (n=149) cases were preventable; 77.7% (n=171) of DRPs were assessed as minor; 22.3% (n=49) as moderate. The high percentage of preventable DRPs emphasizes the importance of providing more training to healthcare professionals in the prescribing and use of medicines in children to minimize the risk of DRPs [7].
A study was conducted to observe the pharmacist clinical knowledge about DRPs and the extent to which they participate in reducing the incidences of DRPs. A questionnaire-based survey was conducted among hundred pharmacists selected by random sampling in different health care settings and according to data collected it was found that different types of DRPs were identified by Pharmacists but only 41% of Pharmacists reported these DRPs and 37% of pharmacists intervened to reduce the incidences of DRPs [8].
All the above fact reported gave us a wide scope for performing a study on drug related problems.
Conclusion
Clinical pharmacist as a part of multidisciplinary team is associated with a substantially lowering rate of adverse drug event caused by medication errors, drug interactions, and drug incompatibilities, under dosing and overdosing and improve patient safety and outcome, reduce costs, and provide quality of care in pediatric population. The clinical pharmacist shall function as a mediator between the physician and the patients to ensure rational use of drugs.
Ziai M (2004) The Joys of Pediatrics. American Academy of Pediatrics 1-5. [ Ref ]
Walker R and Whittlesea C (2011) Clinical pharmacy and Therapeutics. Churchill Livingstone. [ Ref ]
Gonzalez G, Caroca CM , Paris E (1998) Adverse drug reactions (ADRs) in hospitalized pediatric patients-A prospective study. International Journal of Pharmacology and Therapeutics 36: 530-533. [ Ref ]
Pharmaceutical Care Network Europe (PCNE) (2006) Looking for the different versions of the PCNE-DRP classification? Look at the bottom of this page [https://www.pcne.org/working-groups/2/drug-relatedproblem- classification] 20 July 2016. [ Ref ]
Wilton L, Charles CHL, Kwong BYS, Leung S, Ian CK, et al. (2013) Epidemiology and potential risk factors of drug-related problems in Hong Kong paediatric wards. British Journal of Clinical Pharmacology 77: 873-879. [ Ref ]
Sabry N, Farid S, Dawoud D (2014) Drug related problems in cardiac children. Minerva pediatrica 68: 89-35. [ Ref ]
Rashed A, Tomlin A, Jackman J, Neubert A, Wilton L, et al. (2012) Epidemiology and potential associated risk factors of drug related problems in hospitalized children in UK. BMJ 98: 1-3. [ Ref ]
Jamal I, Amin F, Jamal A, Saeed A (2015) A Pharmacist’s interventions in reducing the incidences of drug related problems in any practice setting. International Current Pharmaceutical Journal 4: 347-352. [ Ref ]