Journal Name: Journal of Clinical Case Reports and Trials
Article Type: Case Report
Received date: 03 October 2019
Accepted date: 15 October 2019
Published date: 18 October 2019
Citation: Gholmieh L, Jalbout YA (2019) Laparoscopic Diaphragmatic Hernia Repair in a Patient with Bullous Emphysema under General Anesthesia: A Case Report. J Clin Case Rep Trials. Vol: 2, Issu: 2 (22-25).
Copyright: © 2019 Gholmieh L. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The anesthetic management of patients with bullous emphysema undergoing laparoscopic surgery is challenging due to the lifethreatening complications that might occur from bullae rupture during anesthesia induction, maintenance, and emergence. However, successful management can be achieved when the proper measures are taken. Intraoperatively, nitrous oxide should be avoided, lungs ventilated using pressure-control ventilation, peak inspiratory pressure maintained at or around 15 cm H2O, frequent auscultations performed and arterial blood gases taken repetitively. A chest tube should be readily available inside the operating theatre with the on call cardiothoracic surgeon in close reach.
Keywords
Bullous emphysema, laparoscopic surgery, general anesthesia.
Abstract
The anesthetic management of patients with bullous emphysema undergoing laparoscopic surgery is challenging due to the lifethreatening complications that might occur from bullae rupture during anesthesia induction, maintenance, and emergence. However, successful management can be achieved when the proper measures are taken. Intraoperatively, nitrous oxide should be avoided, lungs ventilated using pressure-control ventilation, peak inspiratory pressure maintained at or around 15 cm H2O, frequent auscultations performed and arterial blood gases taken repetitively. A chest tube should be readily available inside the operating theatre with the on call cardiothoracic surgeon in close reach.
Keywords
Bullous emphysema, laparoscopic surgery, general anesthesia.
Introduction
Many patients with emphysema will develop cystic air spaces in the lung parenchyma known as bullae [1]. Giant bullous emphysema needs specific considerations in non thoracic surgeries as these patients are at high risk for developing pneumothorax at any time from induction of anesthesia till extubation.
We here by present the anesthetic management of a patient with giant bullae who underwent laparoscopic diaphragmatic hernia repair under general anesthesia. Written consent was obtained from the patient before writing this case report.
Case Report
This is a case of a 59-year-old male who presented for laparoscopic diaphragmatic hernia repair under general anesthesia.
The patient is known to have idiopathic thrombocytopenic purpura, diagnosed in 2000 status post partial splenectomy in 2001 followed by complete splenectomy in 2008. The latter was complicated by bleeding, causing further drop in his platelet count. Accordingly, the patient was started on Rituximab, last dose was in 2014.
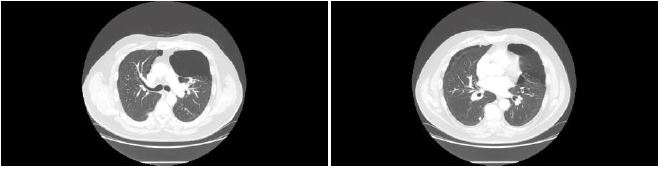
The patient, never a smoker with negative family history for respiratory disease, was diagnosed with paraseptal bullous emphysema in 2001. A recent chest High Resolution Computerized Tomography revealed diffuse paraseptal and centrilobular destructive emphysematous changes with a large peripheral air bullous at the anterior segment of the left upper lobe grossly measuring 9.3x9 cm and a 9 mm nodule at the apical segment of the right lower lobe [Figure 1].
Figure 1:HRCT chest showing multiple bullae.
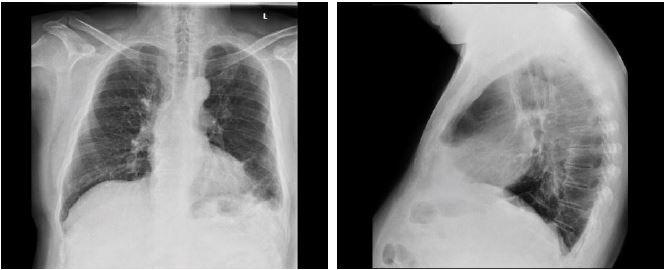
Figure 2:PA and lateral views of the chest showing left sided pleural effusion with basal atelectatic bands
He also has obstructive sleep apnea, untreated because of his bullae, and hypertension managed by daily valsartan 160mg.
On presentation for preoperative clearance, his vital signs were stable with a blood pressure of 119/80mmHg, heart rate of 66beats/min, and oxygen saturation of 98% on room air. On physical exam, expiratory wheezes were noted.
Blood work-up was normal, electrocardiogram showed premature ventricular contractions, and chest X-ray revealed left sided pleural effusion with subsequent atelectatic changes at the left base with blunting of the ipsilateral hemidiaphragm [Figure 2].
Blood work-up was normal, electrocardiogram showed premature ventricular contractions, and chest X-ray revealed left sided pleural effusion with subsequent atelectatic changes at the left base with blunting of the ipsilateral hemidiaphragm [Figure 2].
Pulmonary function testing revealed a forced expiratory volume at 1 second (FEV1) of 74% of predicted, ratio of forced expiratory volume at 1 second to forced vital capacity (FEV1 /FVC) of 71% of predicted, no air trapping, and no response to bronchodilators with a DLCO (Diffusing capacity of the lungs for carbon monoxide) of 75%. Room air ABGs analysis showed a pH of 7.34, pCO2 of 42 mmHg, pO2 of 81 mmHg, HCO3- of 22.7 meqL-1, and SaO2 of 95%. A pulmologist was consulted to optimize the patient’s lung function preoperatively and identify the possible risks. A thoracic surgeon was contacted and kept on hold for adequate management of any intraoperative emergency.
Prior to entering the operating room, the patient received one session of inhaled ipratropium bromide/salbutamol, one session of inhaled budesonide by nebulizers and 40mg of intravenous methylprednisolone.
In the induction room, a 20G intravenous cannula was inserted. Once inside the operating room, a five-lead ECG, noninvasive blood pressure monitoring and pulse oximetry were applied as part of routine monitoring. Remi-fentanyl and Dexmetetomidine infusions at a rate of 0.065 mcg/kg/min and 0.5mcg/kg/hr were started, respectively, 10 minutes before induction.
The patient was preoxygenated with 100% oxygen at a flow of 10L/min. Once ETOz (end tidal oxygen) > 85%, rapid sequence induction was initiated with 200mg of intravenous propofol and 100mg of intravenous succinylcholine. An 8.0 cuffed endotracheal tube was placed using a video laryngoscope. The cuff was inflated with 7ml of air with the pressure checked not to exceed 20cmH2O. A peripheral nerve stimulator was placed on the medial side of the right forearm aiming at detecting the level of ulnar nerve paralysis as measured through the adductor pollicis muscle activity. After obtaining a train-of-four (TOF) count of 4, 20mg of cisatracurium were administered. Anesthesia was maintained with FiO2 0.6 and sevoflurane (1.4-1.8%). The ventilation mode was adjusted to pressure-controlled with the Peak airway Pressure maintained at or below 15. A nasogastric tube and an esophageal temperature probe were inserted. Both Remi-Fentanyl and Dexmedetomidine infusions were continued throughout the operation with rates adjusted according to patient’s intraoperative requirements.
Tidal volume was kept at 500mL with peak inspiratory pressure (PIP) of 14cm H2O, respiratory rate (RR) of 13 breaths/minute and an end tidal carbon dioxide (ETCO2) of 29 mmHg. As pneumoperitoneum was created, the intrabdominal pressure was maintained at 12mmHg, the PIP and RR were increased to 16 and 15 respectively to maintain a tidal volume of 460-470ml throughout the entire operation and prevent the ETCO2 from exceeding 35 mmHg. Frequent chest auscultations were performed. Saturation was maintained at or above 96%. The procedure ended without any adverse events.
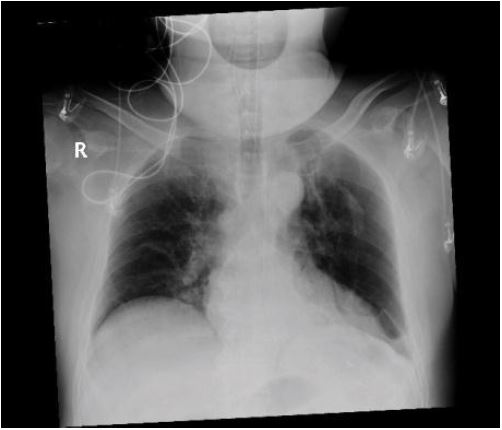
Just prior to extubation, a portable chest X-ray was obtained showing no evidence of pneumothorax or pneumomediastinum rather a persistent left sided pleural effusion with atelectatic changes [Figure 3].
Figure 3:Portable chest x-ray pre-extubation showing no pneumothorax.
Post extubation, the patient was transferred to the PACU for close-up monitoring and pain management using intravenous morphine titration. Repeat portable chest X-ray showed no evidence of pneumothorax.
The patient was discharged on post-operative day 3 with an uneventful overall stay.
Discussion
Emphysema is a destructive process of the alveolar structures. Pulmonary “bullae” are pathologically dilated air spaces distal to the terminal bronchiole measuring more than 2 cm in diameter in the distended state [2]. Generally, pulmonary emphysema is classified into three types based on lobular anatomy: centrilobular emphysema, panlobular emphysema, and paraseptal emphysema [3].
The development of bullous emphysema has been associated with tobacco use, α1-antitrypsin deficiency, marijuana and cocaine with the latter two seen mostly in young patients with no other risk factors [4].
Rupture of giant bullae during anesthesia induction and positive pressure ventilation can lead to potential life-threatening situations such as pneumothorax, pneumopericardium, hypoxemia, and death [5].
Ventilatory strategies used in patients with bullous emphysema to prevent the potential risks of ventilator-induced lung injury (VILI) have not been standardized [6]. Most authorities recommend avoidance of nitrous oxide use since it causes rapid enlargement of the bullae. During the initial stages of administration, the rate of nitrous oxide transfer from blood to air-filled bullae may be 34 times greater than the rate of nitrogen removal. The increased number of nitrous oxide molecules in the bullae results in an increase in its volume, if compliant, or a rise in pressure, in a noncompliant air space [7].
Spontaneous ventilation is recommended throughout the entire procedure7. The reason is that bullae observed in inspiration and expiration don’t change size to any appreciable degree and the change that does occur is always in phase (i.e growth during inspiration and emptying during expiration) [8]. During positive pressure ventilation, bullae are exposed directly to filling pressures that produce local positive end expiratory pressure within them8 leading to their rapid enlargement making them vulnerable to rupture8. However, in longer-duration surgeries, maintenance of spontaneous ventilation and appropriate depth of anesthesia at the same time might be difficult [2]. Accordingly, Hillier et al. believed that pressure-controlled or pressure-limited ventilation is preferred over volume-controlled ventilation [9].
Although the use of PEEP in such patients is controversial, Zhong et al. suggested that low PEEP levels, of 5 mmHg, provided adequate oxygenation and minimized VILI in patients with bullous emphysema undergoing cardiac surgery [6].
The best ventilation practice in bullectomy includes PIP <20 cm H2O, tidal volume of 5-6ml/kg of ideal body weight, and early extubation [10].
It is important to decrease emergence phenomena in these patients, mostly coughing, to avoid intrathoracic pressure increase that might lead to bullae rupture and the occurrence of a pneumothorax, whose severity cannot be predicted.
Most published articles explain the anesthetic management of patients undergoing surgery for bullous emphysema treatment known as lung volume reduction surgery. However, the literature lacks the anesthetic considerations in patients with bullous emphysema undergoing non-thoracic surgery and is scarce on data regarding those considerations during laparoscopic surgery.
Dutta et al were the only to publish a case report about the aforementioned topic. In their paper, the anesthesiologist carried the same precautions we did with our patient. Nitrous oxide was avoided, intraabdominal pressure was kept at 10mmHg, PIP maintained at 15cm H₂O or below, frequent auscultations were performed, and ABGs were taken on a regular basis [11]. When it comes to patient positioning, laparoscopic diaphragmatic hernia repair requires placing patient in reverse trendelenburg position with slight elevation of the right lateral side. This position does not itself cause respiratory compromise [11].
In conclusion, the anesthestic management of patients with bullous emphysema is challenging and adding laparoscopic surgery to the picture aggravates the situation. None the less, successful management of such patients can be achieved as evidenced by already published Dutta et al. case report and our own.
Conflicts of Interest
None.
Mottaghi K, Asadi S, Safari F, Nashibi M (2016) Anesthesia management of bullous emphysema in patient candidate for craniotomy. Annals of Anesthesiology and Critical Care. 1: e10190. [ Ref ]
Fletcher CM (1959) Terminology, Definitions, and Classification of Chronic Pulmonary Emphysema and Related Conditions: A Report of the Conclusions of a Ciba Guest Symposium. Thorax. 14: 286-299. [ Ref ]
Takahashi M, Fukuoka J, Nitta N, Ryutaro Takazakura, Yukihiro Nagatani, et al. (2008) Imaging of pulmonary emphysema: A pictorial review. International Journal of Chronic Obstructive Pulmonary Disease. 3: 193-204. [ Ref ]
Johnson M, Smith R, Morrison D, Laszlo G, White R (2000) Large lung bullae in marijuana smokers. Thorax. 55: 340-342. [ Ref ]
Conacher ID (1997) Anaesthesia for the surgery of emphysema. British Journal of Anaesthesia. 79: 530-538. [ Ref ]
Zhong H, Wang W, Zhao B, Yang T (2016) Permissive hypercapnia combined with low-level PEEP in bullous emphysema patient undergoing cardiac surgery: a case report and literature review. International Journal of Clinical and Experimental Medicine. 9: 4680-4684 [ Ref ]
G Caseby N (1981) Anaesthesia for the patient with coincidental giant lung bulla: a case report. Canadian Journal of Anesthesia. 28: 272-276. [ Ref ]
Chakravarthy M, Jawali V (2007) Differential ventilation with spontaneous respiration for bilateral emphysema. Asian Cardiovascular and Thoracic Annal. 15: 35-37. [ Ref ]
Hillier J, Gillbe C (2003) Anaesthesia for lung volume reduction surgery. Anaesthesia. 58: 1210-1219. [ Ref ]
Myles PS, Moloney J (1994) Anesthetic management of a patient with severe bullous lung disease complicated by air leak. Anaesthesia and Intensive Care Journal. 22: 201-203. [ Ref ]
Dutta B Gangaprasad (2012) Anaesthetic management of a case of giant pulmonary bulla undergoing laparoscopic cholecystectomy. Journal of Anesthesia and Clinical Research. 3: 214. [ Ref ]