Journal Name: Journal of Clinical Case Reports and Trials
Article Type: Case Report
Received date: 12 October, 2018
Accepted date: 23 October, 2018
Published date: 30 October, 2018
Citation: Laali A, Minooeifar J, Ebrahimi M, Sam’e M (2018) Pneumocystis Pneumonia in a Patient with Lichen Planus. J Clin Case Rep Trials. Vol: 1, Issu: 2 (22-24).
Copyright: © 2018 Laali A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Lichen planus (LP) is a chronic mucocutaneous disorder of the stratified squamous epithelium that affects oral and genital mucous membranes, skin, nails, and scalp [1]. LP is as a cutaneous and mucosal eruption is usual in middle-aged patients with 30-60 years of age and is more common in females, which rarely reveal with only oral or nail findings [2].
The primary aims of LP treatment are the reduction of painful symptoms, oral mucosal lesions, the risk of oral cancer, and the maintenance of appropriate oral hygiene [3]. Up to now, diverse therapies are described for LP including drug therapy, surgery, psoralen with ultraviolet light A (PUVA), and laser [4]. Different drugs have been used in the form of topical and intravenous for the LP treatment [5,6]. Systemic corticosteroids and steroid-sparing agents can be used to treat dermatologic conditions like LP [7]. Although these immunomodulatory medications have the possibility to decrease morbidity and mortality; its use accompanied with some risk [8]. Immunosuppression effect of steroids may predispose patients to infection with opportunistic microorganisms [9], such as Pneumocystis jiroveci (previously P carinii), which can cause potentially life-threatening pneumonia [10]. Thus, prophylactic agents must be considered as a crucial part of treatment for LP. In this study, we reported a case with 4 years steroid treatment period who was involved with pneumocystis pneumonia.
Keywords
Lichen planus, Pneumonia, Pneumocystis jiroveci, Tumor cells.
Introduction
Lichen planus (LP) is a chronic mucocutaneous disorder of the stratified squamous epithelium that affects oral and genital mucous membranes, skin, nails, and scalp [1]. LP is as a cutaneous and mucosal eruption is usual in middle-aged patients with 30-60 years of age and is more common in females, which rarely reveal with only oral or nail findings [2].
The primary aims of LP treatment are the reduction of painful symptoms, oral mucosal lesions, the risk of oral cancer, and the maintenance of appropriate oral hygiene [3]. Up to now, diverse therapies are described for LP including drug therapy, surgery, psoralen with ultraviolet light A (PUVA), and laser [4]. Different drugs have been used in the form of topical and intravenous for the LP treatment [5,6]. Systemic corticosteroids and steroid-sparing agents can be used to treat dermatologic conditions like LP [7]. Although these immunomodulatory medications have the possibility to decrease morbidity and mortality; its use accompanied with some risk [8]. Immunosuppression effect of steroids may predispose patients to infection with opportunistic microorganisms [9], such as Pneumocystis jiroveci (previously P carinii), which can cause potentially life-threatening pneumonia [10]. Thus, prophylactic agents must be considered as a crucial part of treatment for LP. In this study, we reported a case with 4 years steroid treatment period who was involved with pneumocystis pneumonia.
Keywords
Lichen planus, Pneumonia, Pneumocystis jiroveci, Tumor cells.
Case Study
A 48-year-old woman was admitted in our outpatients’ facility with a 3-week history of fever, chill, dyspnea and productive cough. She was treated as community-acquired pneumonia (CAP) with different antibiotics like Azithromycin and Levofloxacin, but she had no improvement. Past medical history includes Lichen planus. Patient medications include prednisone 15 mg daily and mycophenolate mofetil (MMF) 1000 mg daily (with a history of 4 years consumption) for LP. She was reported as having no known drug allergies, vomiting, dysuria and sneezing symptom or contact with an infectious source.
The patient was admitted and undergone comprehensive assessment and clinical examination revealed nothing more than a crackle in lung auscultation. Laboratory findings are illustrated in table 1. ABG was normal (Table 1).
Table 1:Laboratory test results.
| Test | Result | Unit | Ref. value | Flag |
|---|---|---|---|---|
| WBC | 3300 | Cu.mm | 4000-11000 | Low |
| RBC | 3.78 | mil/Cu.mm | 4.2-5.6 | Low |
| Hgb | 11.3 | g/DL | 11.7-16 | Low |
| Neutrophil | 74 | % | 55-75 | --- |
| Lymphocyte | 22 | % | 20-40 | --- |
| LDH | 337 | U/L | <240 | High |
| PPD | 1 | mm | --- | --- |
| BAL MTB-PCR | Neg. | --- | --- | --- |
| BAL MTB-smear | Neg. | --- | --- | --- |
| HIV Ab | Neg. | --- | --- | --- |
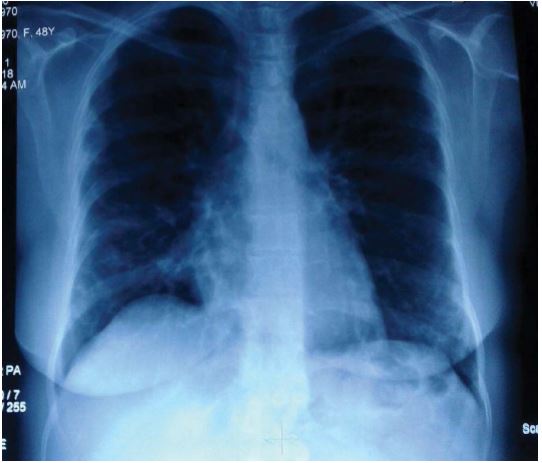
Figure 1:CXR shows bilateral patchy infiltration.
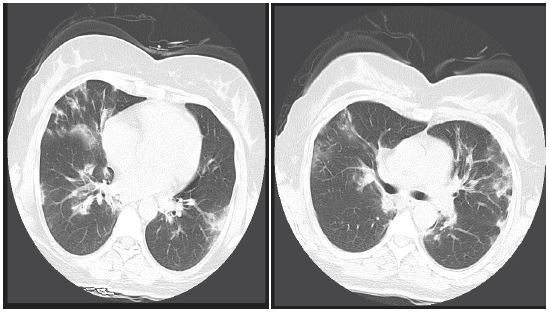
Figure 2and 3:Lung CT scan shows patchy infiltration.
CXR revealed bilateral patchy infiltration (Figure 1) and Spirometry test showed no restrictive or obstructive disorder. A lung CT scan was performed and patchy consolidation, especially in the periphery, was reported (Figures 2 and 3).
Fiberoptic bronchoscopy (FOB) and bronchoalveolar lavage (BAL) were done. BALs were analyzed for microbial and fungal examination and cytological evaluation which were negative for Acid fast bacilli, Nocardia and Tumor cells but were positive for Pneumocystis jiroveci PCR. She was diagnosed with pneumocystis pneumonia, with the causative agent suspected to be long-term use of steroids and mycophenolate mofetil. The treatment was started with co-trimoxazole (15 mg TMP/kg/day)//IV for 2 weeks. After 2 weeks the patient was discharged with a good condition. Prophylactic co-trimoxazole was started for her with a dose of SS 480 mg/ daily.
Discussion
Pneumocystis is a kind of opportunistic fungus that is commonly exist in the nature. Pneumocystis jiroveci is the major underlying organism for PCP on immunocompromised hosts which is transmitted to its source through the airborne route [11]. COPD patients usually experience colonization of the microorganism. In people with HIV, PCP has less severity and slow progression but morbidity and mortality in Non- HIV are high [12]. Non- HIV patients who are assumed predisposed for the disease are those with malignancy, immunosuppressant drug consumption, organ transplantation (solid organ and stem cells) and malnutrition. Rare cases were reported among non-immunocompromised patients [13].
PCP clinical manifestation includes dry cough, shortness of breath, bilateral interstitial infiltration, fever and hypoxemia. The principle diagnostic methods are bronchoalveolar lavage and sputum culture [14]. Up to 90% of patients with PCP, have a history of corticosteroids consumption [15]. Moreover, mycophenolate mofetil and low dose corticosteroids might be another predisposing factor [16]. In our case, co-consumption of these drugs had led to PCP, while patient did not used to medicate with prophylaxis regimen.
The benefit of prophylaxis for Pneumocystis jiroveci pneumonia (PJP) is well documented in immunocompromised patients, especially those with HIV and/or AIDS. The role of co-trimoxazole prophylaxis in non- HIV patients on chronic steroids remains controversial [17]. Some researchers suggest implementation of a prophylaxis regimen for patients who use 20 mg prednisone per day for one month [18]. Although prophylaxis recommendations to all recipients of more than 20 mg of prednisone may also involve patients at low risk for PCP [19].
The drug of choice for prophylaxis is daily use of cotrimoxazole (DS/SS). Although use of SS form may cause lower side effects. Alternative regimens are co-trimoxazole DS (triple doses weekly), Dapsone, pyrimethamine and leucovorin, pentamidine, Atovaquone, Atovaquone plus pyrimethamine and leucovorin
Conclusion
Routine use of prophylaxis regimen is not still clear for non-HIV PCP infected patients. By presenting this case we meant to highlight the risk among immunosuppressant consumer patients.
Canto AM, Müller H, Freitas RR, Santos PS (2010) Oral lichen planus (OLP): clinical and complementary diagnosis. An Bras Dermatol. 85: 669-675. [ Ref ]
Krupaa RJ, Sankari SL, Masthan K, Rajesh E (2015) Oral lichen planus: An overview. J Pharm Bioallied Sci. 7: S158-161. [ Ref ]
Le Cleach L, Chosidow O (2012) Lichen planus. New Eng J Med. 366: 723-732. [ Ref ]
Al-Hashimi I, Schifter M, Lockhart PB, Wray D, Brennan M, et al. (2007) Oral lichen planus and oral lichenoid lesions: diagnostic and therapeutic considerations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 103: 1-12. [ Ref ]
Scully C, Carrozzo M (2008) Oral mucosal disease: Lichen planus. Br J Oral Maxillofac Surg. 46: 15-21. [ Ref ]
Thongprasom K, Dhanuthai K (2008) Steriods in the treatment of lichen planus: a review. J Oral Sci. 50: 377-385. [ Ref ]
Moles GM, Bravo M, Ruiz GL, Ramos P, Montoya GJ, et al. (2018) Outcomes of oral lichen planus and oral lichenoid lesions treated with topical corticosteroid. Oral Dis. 24: 573-579. [ Ref ]
Bombeccari GP, Guzzi G, Tettamanti M, Giannì AB, Baj A, et al. (2011) Oral lichen planus and malignant transformation: a longitudinal cohort study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 112: 328-334. [ Ref ]
Marable D, Bowers L, Stout T, Stewart C, Berg K, et al. (2016) Oral candidiasis following steroid therapy for oral lichen planus. Oral Dis. 22: 140-147. [ Ref ]
Lee SJ, Flowers ME (2008) Recognizing and managing chronic graftversus- host disease. ASH Education Program Book. 2008: 134-141. [ Ref ]
Dumoulin A, Mazars E, Seguy N, Gargallo-Viola D, Vargas S, et al. (2000) Transmission of Pneumocystis carinii disease from immunocompetent contacts of infected hosts to susceptible hosts. Eur J Clin Microbiol Infect Dis. 19: 671-678. [ Ref ]
Bennett JE, Dolin R, Blaser MJ (2014) Principles and practice of infectious diseases. Else Heal Sci. 8: 3904. [ Ref ]
Sharma S, Wills R (2011) A Rare Case of Pneumocystis Pneumonia. Am J Clin Med. 8: 94-97. [ Ref ]
Wilkin A, Feinberg J (1999) Pneumocystis carinii pneumonia: a clinical review. Am Fam Physician. 60: 1699-1708. [ Ref ]
Kelly DM, Cronin S (2013) PCP prophylaxis with use of corticosteroids by neurologists. Pract Neurol. 13: 000727. [ Ref ]
Wan QJ, Hu HF, He YC, Luan SD, Chen HT, et al. (2015) Severe pneumonia in mycophenolate mofetil combined with low-dose corticosteroidstreated patients with immunoglobulin A nephropathy. Kaohsiung J Med Sci. 31: 42-46. [ Ref ]
Liebling M, Rubio E, Ie S (2015) Prophylaxis for Pneumocystis jiroveci pneumonia: is it a necessity in pulmonary patients on high-dose, chronic corticosteroid therapy without AIDS?. Expert Rev Respir Med. 9: 171- 181. [ Ref ]
Kovacs JA, Masur H (2009) Evolving health effects of Pneumocystis: one hundred years of progress in diagnosis and treatment. Jama. 301: 2578- 2585. [ Ref ]
Overgaard UM, Helweg-Larsen J (2007) Pneumocystis jiroveci pneumonia (PCP) in HIV-1-negative patients: a retrospective study 2002–2004. Scand J Infect Dis. 39: 589-595. [ Ref ]