Journal Name: Journal of Drug Development and Delivery
Article Type: Research
Received date: 24 April 2019
Accepted date: 22 May 2019
Published date: 29 May 2019
Citation: Begum A, Sridhar R (2019) Formulation Development and In-vitro Evaluation of Sitagliptin Floating Tablets. J Drug Dev Del Vol: 2, Issu: 1 (25-30).
Copyright: © 2019 Begum A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The present research work done with an objective of preparation and evaluation of floating tablets of Sitagliptin drug with hydroxypropylene methyl cellulose (HPMC), Xanthum Gum and Guar gum polymers. Floating tablets were based on effervescent approach using sodium bicarbonate a gas releasing agent. Direct compression method was used in present study for preparation of tablets. Effect of polymers was evaluated by studying swelling properties and floating time. In-vitro drug release profile indicates that sustained nature increased by increasing the concentration of polymer. The formulation containing Guar gum with 40% concentration is optimized as it showed drug release up to 12hrs. Optimized formulation with 35mg of floating agent per tablet showed desired floating lag time.
Keywords:Floating tablets, Xanthum gum, Guar gum, Sitagliptin, HPMC, Floating lag time.
Abstract
The present research work done with an objective of preparation and evaluation of floating tablets of Sitagliptin drug with hydroxypropylene methyl cellulose (HPMC), Xanthum Gum and Guar gum polymers. Floating tablets were based on effervescent approach using sodium bicarbonate a gas releasing agent. Direct compression method was used in present study for preparation of tablets. Effect of polymers was evaluated by studying swelling properties and floating time. In-vitro drug release profile indicates that sustained nature increased by increasing the concentration of polymer. The formulation containing Guar gum with 40% concentration is optimized as it showed drug release up to 12hrs. Optimized formulation with 35mg of floating agent per tablet showed desired floating lag time.
Keywords:Floating tablets, Xanthum gum, Guar gum, Sitagliptin, HPMC, Floating lag time.
Introduction
All the pharmaceutical products designed for systemic delivery through the oral route of administration, different type of deliveries such as Sustained Release SR, Controlled Release CR, & Immediate Release IR and the design of dosage form (SD / liquid), developed within the intrinsic characteristics of GI physiology [1].
The successful development of Oral drug delivery system ODDS consists of basic understanding like:
(i) Pharmaco kinetics & dynamics & Physicochemical characteristics of the drug.
(ii) The anatomic & physiologic characteristics of the GIT.
(iii) mode of the dosage form to be designed.
Difficulties conventional oral controlled dosage [2]
1. The short gastric retention time (GRT).
2. Unpredictable gastric emptying time (GET).
Gastro retentive drug delivery system GRDDS
Complete information about GI dynamics like as gastric emptying, small intestine transit, colonic transit, etc. is the key for the designing of oral controlled release dosage forms. The rate & extent of drug absorption from different sites of GI tract and factors that govern the absorption further assist the design of dosage form.
GRDDS
Different types of methods were used to enhance the retention of orally administered forms into the stomach: Floating method, Extending method, Bioadhesive method, Modified shape method, High-density method and other delayed gastric-emptying devices.
| Ingredients (mg) | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 |
|---|---|---|---|---|---|---|---|---|---|
| SITAGLIPTIN | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| HPMC | 105 | 122.5 | 140 | -- | -- | -- | -- | -- | -- |
| XANTHUM GUM | -- | -- | -- | 70 | 87.5 | 140 | -- | -- | -- |
| GUAR GUM | -- | -- | -- | -- | -- | -- | 70 | 87.5 | 140 |
| MCC | 42 | 24.5 | 7 | 77 | 59.5 | 7 | 77 | 59.5 | 7 |
| NaHCo3 | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 | 35 |
| Citric acid | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 |
| Mg. Stearate | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Total Weight | 350 | 350 | 350 | 350 | 350 | 350 | 350 | 350 | 350 |
Table 1: Formulations of Sitagliptin floating tablets.
Advantages of Floating drug delivery systems FDDS [3,4]: Floating technique was modified technology with gastric retentive behavior and it contains no. of advantages.
These advantages include:
1. improved bioavailability.
2. SR reduced frequency of dosing
3. Site specific therapy for GIT.
4. Minimize adverse activity at the colon.
5. Reduced variations of drug conc.
Disadvantages of FDDS:
1. The drugs that are unstable in the acidic environment of the stomach are not suitable candidates to be incorporated in the systems.
2. These are requiring a great concentration of fluid in the stomach for drug delivery to float and work efficiency.
3. Not appropriate for drugs that have solubility / stability problem in GIT.
Types offdds [4]:Based on the method of buoyancy, two different technologies have been utilized in development of FDDS which are:
A. Effervescent system:
It was further separated into 2 types.
I. Gas liberating systems
II. Volatile Liquid/Vacuum Containing Systems.
B. Non-Effervescent system:[5]
The various types of this system are as:
• Single Unit Floating dosage system
• Multiple Unit Floating dosage system
Materials and Methods
Materials
Sitagliptin was used as active agent and it is gifted sample from Chandra labs, Hyderabad. Polymers like HPMC, Guar gum, Xanthum gum and floating agent Sodium bicarbonate, Citric acid, filler like Micro crystaline cellulose were laboratory grade reagents.
Methodology
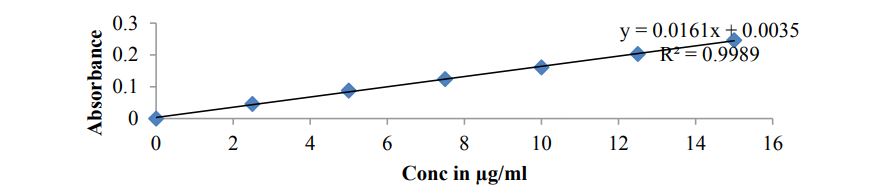
Preparation of calibration curve for Sitagliptin in 0.1N HCL: Prepare 2.5, 5, 7.5, 10, 12.5 and 15 μg/mL concentrations of Sitagliptin solution in 0.1N HCL and note absorbance against blank at 288nm using UV absorption spectrophotometer.
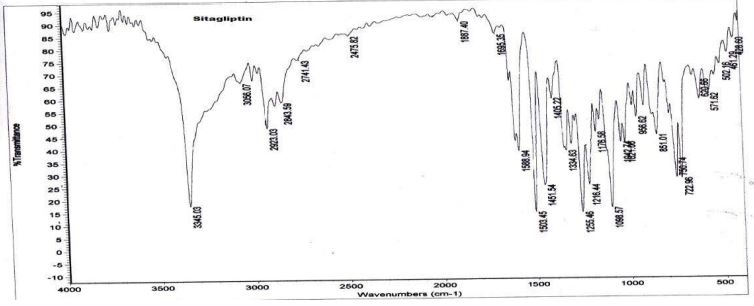
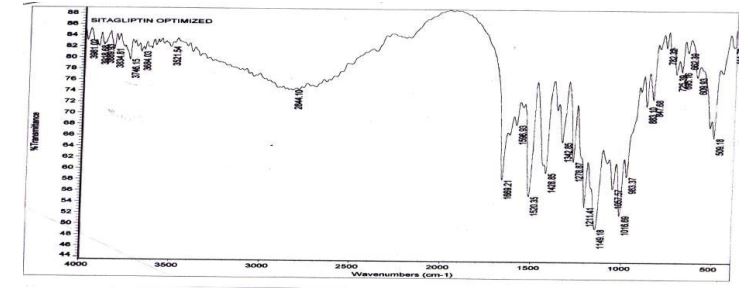
Drug and Excipients compatibility studies using FTIR: In this FTIR the drug functional groups were identified and Compatibility was observed of these excipients with Sitagliptin.
Sitagliptin floating tablets Formulation by direct compression method
Insert (Table 1)
Pre-formulation studies: Pre formulation studies include Description, solubility, melting point of drug and flow properties of formulation blends before punching. Flow properties like Bulk density, Tapped density, Angle of repose, Compressibility index and Hausner’s ratio were studied.
Post compression parameters: Prepared tablet formulations were studied for Thickness, Hardness, Friability, weight variation, swelling index, Buoyancy studies and In-vitro drug release studies.
Kinetic Analysis of in-Vitro Release Rates of Floating Tablets of Sitagliptin [6]: The results of in vitro release profile obtained for all the formulations were plotted in modes of data treatment as follows,
1. Zero order kinetic model
2. 1st order kinetic model
3. Higuchi’s model
4. Korsmeyer equation / Peppa’s model [7,8]
Results and Discussion
Pre formulation results
Description: These tests were performed the results were illustrated in the following table and the results were found as per specifications table 2.
Solubility: These tests were performed and the results are illustrated in the table 3.
Melting Point: This test is performed the result was as per specification only and result illustrated in the following table 4.
Calibration curve of Sitagliptin in 0.1N HCL
Table 5, Figure 1
Fourier transformer infrared spectroscopy FTIR
By correlating Sitagliptin peaks of pure drug spectrum with physical- mixtures of the optimized formulation it was found that the drug is compatible with the formulation components (Figures 2 and 3).
Fourier transformer infrared spectroscopy FTIR
Table 6
Post compression parameters evaluation for floating tablets
Table 7 and 8
In vitro dissolution studies for floating tablets in 0.1N HCL
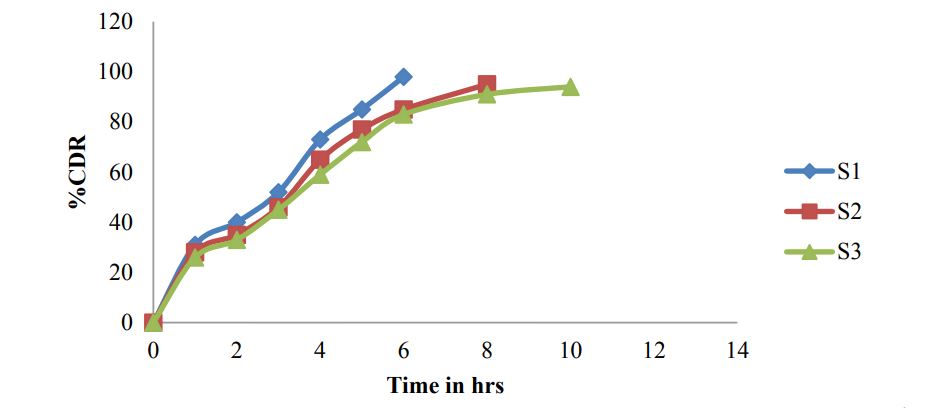
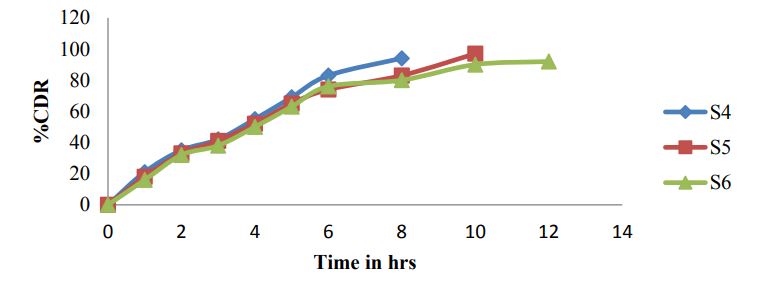
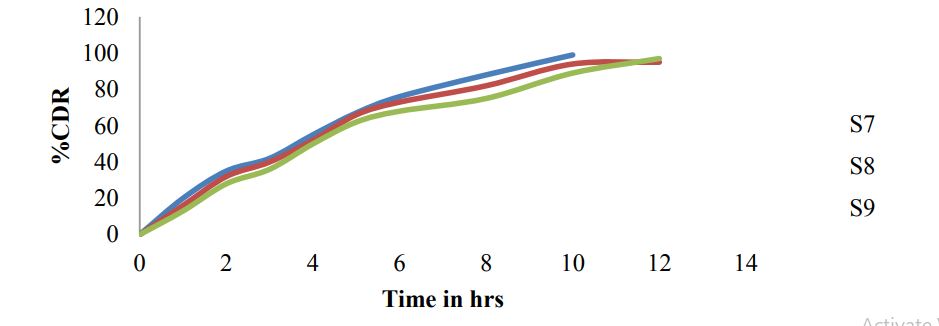
For gastro retentive formulations generally 0.1N HCL was used as dissolution medium and for present formulations 900ml 0.1N HCL, USP Type 2 paddle apparatus, and 5ml samples were withdrawn for every time point Table 9 and Figures 4,5 and 6.
Drug Release kinetics
Table 10
Summary and Conclusion
Gastroretentive drug delivery is an approach to prolong gastric residence time thereby targeting site-specific drug release in the upper gastrointestinal tract for local or systemic effects. Gastroretentive dosage forms remain in the gastric region for longer period and hence significantly prolong the gastric retention time of drugs.
Gastro retentive dosage form by Guar gum was prepared and to develop a floating tablets of Sitagliptin that could retain in the stomach for longer periods of time delivering the drug to the site of action, i.e., in stomach.
Among those anti-diabetic medications Sitagliptin was more acceptable. Sitagliptin Phosphate is a Dipeptidyl Peptidase-4 (DPP-4) Inhibitor. Sitagliptin competitively inhibits the enzyme dipeptidyl peptidase 4 (DPP-4). This enzyme breaks down the incretins GLP-1 & GIP, GI hormones released in response to a meal. By preventing GLP-1 & GIP inactivation, they are able to increase the secretion of insulin and suppress the release of glucagon by the pancreas. This drives blood glucose levels towards normal.
Formulations prepared using direct compression method. The prepared tablets of all the formulations were evaluated for physical characters tablet thickness, hardness and friability, swelling index, floating lag time, total floating time, drug content and in-vitro drug release.
S9 formulation with Guar gum as polymer showed highest drug release for 12 hrs and good buoyancy results. Optimized formulation dissolution data is studied for kinetic release studies and optimized S9 formulation follows Higuchi model.
Figure 1: Calibration curve plot of Sitagliptin.
| Test | Description |
|---|---|
| Colour | White to off white powder |
| Odour | Free of odour |
Table 2: Table showing the description of Sitagliptin (API).
| Solvents | Solubility |
|---|---|
| Water | soluble |
| Ethanol | soluble |
| Methanol | Slightly Soluble |
| Acetone | soluble |
Table 3: Table showing the Solubility of Sitagliptin (API) in various solvents.
| Material | Melting Point |
|---|---|
| Sitagliptin | 216-219°c |
Table 4: Table showing the melting point of API’s.
| Concentration (µg /ml) | Absorbance |
|---|---|
| 0 | 0 |
| 2.5 | 0.046 |
| 5 | 0.088 |
| 7.5 | 0.124 |
| 10 | 0.161 |
| 12.5 | 0.203 |
| 15 | 0.246 |
Table 5: Calibration Curve Data of Sitagliptin.
| Formulation code | Bulk density (g/cc) | Tapped density (g/cc) | Carr’s Index | Hausner Ratio | Angle of repose(θ) |
|---|---|---|---|---|---|
| S1 | 0.45±0.045 | 0.52 ± 0.09 | 15.60±0.2 | 1.15±0.02 | 28.06± 0.31 |
| S2 | 0.45±0.045 | 0.50 ± 0.07 | 12.23±0.6 | 1.11±0.04 | 27.58± 0.15 |
| S3 | 0.44±0.044 | 0.50 ± 0.09 | 12.58±0.8 | 1.13±0.08 | 28.44± 0.11 |
| S4 | 0.45±0.045 | 0.52 ± 0.04 | 15.19±0.1 | 1.15±0.06 | 28.36± 0.13 |
| S5 | 0.44±0.044 | 0.52± 0.01 | 15.48±0.6 | 1.18±0.08 | 28.52± 0.19 |
| S6 | 0.45±0.045 | 0.51 ± 0.04 | 13.48±0.8 | 1.13±0.09 | 29.32± 0.19 |
| S7 | 0.51±0.045 | 0.59 ± 0.04 | 14.48±0.8 | 1.15±0.09 | 29.69± 0.19 |
| S8 | 0.45±0.045 | 0.50 ± 0.07 | 12.23±0.6 | 1.11±0.04 | 27.58± 0.15 |
| S9 | 0.45±0.045 | 0.52 ± 0.04 | 15.19±0.1 | 1.15±0.06 | 28.36± 0.13 |
Table 6: Pre-compression parameters (S1-S9).
Figure 2: FTIR sprectra of drug Sitagliptin.
Figure 3: FTIR Spectra of optimized floating sitagliptin formulation blend.
Figure 4: Dissoulation graph for S1 – S3.
Figure 5: Dissolution graphs for S4-S6.
Figure 6: Dissolution graphs for S7-S9.
| Formulation Code | Weight Variation (mg) | Thickness (mm) | Hardness (kg/cm²) | Friability (%) |
|---|---|---|---|---|
| S1 | 344±1.36 | 3.28±0.20 | 7.3±0.54 | 0.72±0.41 |
| S2 | 348±2.02 | 3.33±0.22 | 7.5±0.75 | 0.37±0.42 |
| S3 | 350±1.89 | 3.28±0.17 | 7.6±0.45 | 0.40±0.38 |
| S4 | 347±1.99 | 3.16±0.05 | 7.9±0.25 | 0.46±0.36 |
| S5 | 348±2.49 | 3.84±0.17 | 7.3 ±0.44 | 0.32±0.25 |
| S6 | 347±1.99 | 3.92±0.25 | 7.6±0.31 | 0.30±0.17 |
| S7 | 349±0.89 | 3.80±0.80 | 7.6±0.40 | 0.36±0.20 |
| S8 | 350±1.88 | 3.82±0.20 | 7.5±0.55 | 0.31±0.25 |
| S9 | 346±1.15 | 3.98±0.66 | 7.7±0.57 | 0.34±0.36 |
Table 7: Post compression parameters (S1-S9).
| Formulation Code | Drug content (%) | Floating lag time | Swelling index (%) | Floating duration (hrs) |
|---|---|---|---|---|
| S1 | 98.78±0.24 | 46sec | 32.12 | 6.3 |
| S2 | 97.70±0.38 | 52sec | 34.26 | 8.1 |
| S3 | 99.51±0.32 | 59sec | 36.01 | 9.2 |
| S4 | 99.94±0.21 | 4min | 34.95 | 8.3 |
| S5 | 98.42±0.28 | 6min | 37.23 | 10.1 |
| S6 | 98.91±0.23 | 7min | 38.18 | 11.0 |
| S7 | 98.58±0.24 | 12sec | 36.55 | 10.3 |
| S8 | 99.26±0.44 | 28sec | 37.75 | 11 |
| S9 | 99.12±0.32 | 36sec | 39.66 | 12 |
Table 8: Post compression parameters (S1-S9).
| Time (hrs) | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 31 | 28 | 26 | 21 | 18 | 16 | 20 | 16 | 13 |
| 2 | 40 | 35 | 33 | 35 | 33 | 32 | 35 | 32 | 28 |
| 3 | 52 | 46 | 45 | 42 | 41 | 38 | 42 | 40 | 36 |
| 4 | 73 | 65 | 59 | 55 | 52 | 50 | 55 | 52 | 50 |
| 5 | 85 | 77 | 72 | 69 | 65 | 63 | 67 | 66 | 62 |
| 6 | 98 | 85 | 83 | 83 | 74 | 76 | 76 | 73 | 68 |
| 8 | - | 95 | 91 | 94 | 83 | 80 | 88 | 82 | 75 |
| 10 | - | - | 94 | - | 97 | 90 | 99 | 94 | 89 |
| 12 | - | - | - | - | - | 92 | - | 95 | 97 |
Table 9: Dissolution data of formulation S1-S9.
| ZERO | FIRST | HIGUCHI | PEPPAS | |
|---|---|---|---|---|
| % CDR Vs T | Log % Remain Vs T | %CDR Vs √T | Log C Vs Log T | |
| Slope | 7.971202304 | -0.11465774 | 30.35961017 | 1.308499573 |
| Intercept | 11.14686825 | 2.115093439 | -9.78941327 | 0.761137851 |
| Correlation | 0.972099715 | -0.96315767 | 0.985702545 | 0.846467921 |
| R 2 | 0.944977857 | 0.927672697 | 0.971609507 | 0.716507941 |
Table 10: Kinetic values of S9 formulation.
Robinson Jr, Lee VHL (1978) Controlled drug delivery: Fundamentals and Applications. Marcel Dekker, New York.[ Ref ]
Chein YW (1992) Novel Drug Delivery Systems. Marcel Dekker, New York.[ Ref ]
Banker GS, Rhodes CT (1996) Modern Pharmaceutics. Marcel Dekker, New York.[ Ref ]
Vyas SP, Khar RK (2002) Controlled Drug Delivery: Concepts and Advances. Vallabh prakashan, India. [ Ref ]
Yeole PG (2005) Floating Drug Delivery System: Need and Development. Ind J Pharm Sci 67: 265-272. [ Ref ]
Shweta Aurora (2005) Floating Drug Delivery: A Review. AAPS Pharmscitech.: 47: 268-272. [ Ref ]
Desai S, Bolton SA (1993) Floating Controlled Release System: In-vitro and In-vivo evaluation. J Pharm Res 10: 1321-1325.[ Ref ]
Garg S, Sharma S (2003) Gastro retentive Drug Delivery Systems. Pharma tech 3: 160-164.[ Ref ]