Journal Name: Journal of Drug Development and Delivery
Article Type: Research
Received date: 22 August 2019
Accepted date: 02 September 2019
Published date: 09 September 2019
Citation: Shilpa V, Kiran K (2019) Formulation Development and In-vitro Evaluation of Vildagliptin Controlled Release Tablets. J Drug Dev Del Vol: 2, Issu: 2 (17-22).
Copyright: © 2019 Shilpa V. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The overall objective of present study was to develop an oral controlled release (CR) Vildagliptin tablets by using natural polymers like Guar gum, xanthan gum and synthetic polymers like Hydroxy propyl methyl cellulose (HPMC), Ethyl cellulose (EC). Total six different CR formulations were prepared by direct compression method. FTIR studies shows that there is no interaction between drug and excipients. In-vitro dissolution studies were carried out using USP paddle apparatus and drug release data was analysed by using zero order, first order, higuchi, kosmeyer and peppas drug release kinetic models. Formulation F1 containing 10% Guar gum was optimized as it shows drug release up to 12hrs. Optimized formulation drug release follows zero order release kinetics and higuchi type of kinetic model.
Keywords
Vildagliptin, Guar gum, Xanthan gum, EC, Direct compression.
Abstract
The overall objective of present study was to develop an oral controlled release (CR) Vildagliptin tablets by using natural polymers like Guar gum, xanthan gum and synthetic polymers like Hydroxy propyl methyl cellulose (HPMC), Ethyl cellulose (EC). Total six different CR formulations were prepared by direct compression method. FTIR studies shows that there is no interaction between drug and excipients. In-vitro dissolution studies were carried out using USP paddle apparatus and drug release data was analysed by using zero order, first order, higuchi, kosmeyer and peppas drug release kinetic models. Formulation F1 containing 10% Guar gum was optimized as it shows drug release up to 12hrs. Optimized formulation drug release follows zero order release kinetics and higuchi type of kinetic model.
Keywords
Vildagliptin, Guar gum, Xanthan gum, EC, Direct compression.
Introduction
The oral route of drug delivery is one of the most convenient means to administer drug to the human body to obtain the desired therapeutic effect. Though it is a convenient route it provides several challenges to the formulator to design a medication such that it provides the drug in an optimum concentration needed to attain a plasma level of the drug which will fall within the therapeutic window to obtain the desired effect [1].
Controlled release systems provide a slow release of drug over an extended period of time and also can provide some control, this system is successful at maintaining constant drug levels in the target tissue of cells. Sustained release systems include any drug delivery system that achieves slow release of drug over an extended period of time. Extended release pharmaceutical dosage forms that release the drug slower than normal manner at predetermined rate and necessarily reduce the dosage frequency by two folds.
The overwhelming majority of controlled-release systems rely on dissolution, diffusion, or a combination of dissolution and diffusion to generate slow release of a drug. Starting with limited data on a drug candidate for a sustained-release system, such as dose, rate constants for absorption and elimination, and some elements of metabolism, one can compute a desired release-rate for the dosage form, and the amount of drug required [2-6].
Vildagliptin drug is an oral anti-hyperglycemic agent. It inhibits the dipeptidyl peptidase-4 and potentiates the release of insulin, suppresses the glucagon secretion in pancreas. Vildagliptin improves the glycemic control in patients with type 2 diabetes mellitus as monotherapy. Vildagliptin treatment is characterized by weight neutral and lipid neutral effects, less risk of edema and less risk of hypoglycemia.
| Ingredients (%) | F1 | F2 | F3 | F4 | F5 | F6 |
|---|---|---|---|---|---|---|
| Vildagliptin (mg) | 5 | 5 | 5 | 5 | 5 | 5 |
| Guar gum | 10% | 15% | - | - | - | - |
| Ethyl cellulose | - | - | 10% | 15% | ||
| Xanthan gum | 10% | 15% | ||||
| MCC | Qs | Qs | Qs | Qs | Qs | Qs |
| Magnesium Stearate | 2% | 2% | 2% | 2% | 2% | 2% |
| Talc | 2% | 2% | 2% | 2% | 2% | 2% |
| PVPK30 | 5% | 5% | 5% | 5% | 5% | 5% |
| Total weight (mg) | 200 | 200 | 200 | 200 | 200 | 200 |
Table 1: Formulation table.
| INGREDIENTS | Weight | CONCENTRATION (%) |
|---|---|---|
| HPMC E15 | 4gm | 4% |
| Triethyl citrate | 400mg | 0.4 |
| Methanol | 20ml | Quantity sufficient |
Table 2: Coating solution composition.
| Description | Colour white to off whiteOdour free of odour |
| Solubility | Water freely solublepH 6.8 phosphate buffer soluble Methanol solubleDMSO soluble |
| Melting point | 222 – 224°C |
Table 3: Characteristics of vildagliptin (API).
Materials and Methods
Materials
Drug Vildagliptin was gifted sample received from Chandra labs, Hyderabad. Polymers Xanthan gum, Guar gum, HPMC E15 and Ethyl cellulose were purchased from MYL chemicals, Mumbai. Other excipients like MCC, Magnesium stearate, Talc and PVP were purchased from S.D fine chemicals, Mumbai. Reagents used in present research work were laboratory grade.
Pre-formulation studies: Scanning of drug
Drug solution (10μg/ml) was prepared using ethanol as solvent. Drug solution was scanned using UV-Visible spectrophotometer, to determine the λ max for Vildagliptin drug [7].
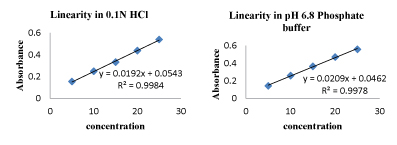
Construction of calibration curve in 0.1N HCL and 6.8 pH phosphate buffers
10 mg of Vildagliptin was dissolved in 10 ml of volumetric flask using respective buffer which gives concentration of 1000 μg/ml. Then 1ml of stock solution was taken and diluted to 100 ml which gives a concentration of 10μg/ml. From this stock solution subsequent dilutions were made in buffer in order to get 5μg/ml, 10 μg/ml, 15 μg/ml, 20 μg/ ml, 25 μg/ml, Absorbance of these solutions were measured at λ max. The linearity plot was obtained for the aliquot concentration of 5, 10, 15, 20; 25μg/ml with the absorbance was seen at λ max.
Drug – excipient compatibility study
IR spectrum is a helpful analytical method utilizes to ensure the chemical interaction between the drug and excipients. Spectrum measurement was passed out by using IR spectrum is a helpful analytical method utilizes to ensure the chemical interaction between the drug and excipients. Spectrum measurement was passed out by using
Formulation of tablets
Tablets prepared by direct compression method. Excipients MCC, Guar gum, Xanthan gum and Ethyl cellulose were weighed and sifted through 40 number mesh. To this blend add Vildagliptin drug and sifted through 18 number mesh. Mix the above blend for 10min and add Magnesium stearate, Talc and PVP K30 and mix properly. This blend was compressed using 9mm round punches [11] Table 1.
Coating with semi-permeable polymer: By the coating missionary system the core tablets were coated. A HPMC E15 solution of in Methanol at a concentration of (4%w/v), containing Triethylcitrate (TEC) at concentration of 10% of w/w of HPMC E15, level of plasticizer (TEC) was used as the coating solution. To the Methanol, HPMC E15 added slowly under proper stirring Table 2.
Coating conditions
Pan rpm : 10-11
Coating solution spray rate : 4-5ml/min
In let temperature : 38°C
Outlet temperature : 28°C
Atomizer pressure : 1.0 kg/cm2
Fan pressure : 1-0.75 kg/cm2
Inlet air blower : 900 cpm
Outlet air blower : 1600 cpm
Pre-compression parameters
Flow properties for all formulations were calculated using mixed blend before punching. Angle of repose, Bulk density & Tapped density, Hausner ratio and Compressibility index (%) were calculated to assess the uniformity of drug and hardness after punching.
Evaluation of tablets
Tablets were evaluated for Hardness, Friability, Weight variation, Drug content uniformity, In-vitro dissolution, release kinetic studies and stability studies.
Results
Pre-formulation studies
Description and characteristics of API: Table 3
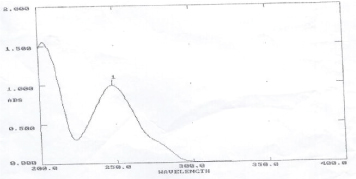
Scanning of API: Figure 1
Calibration curves of vildagliptin: Figure 2
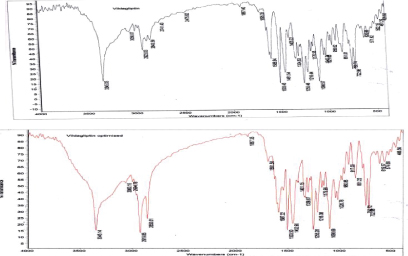
Drug-Excipient Compatibility study (FTIR): Figure 3
FTIR Interpretation: FTIR studies were conducted to appreciate the compatibilities between the drug and different excipients. The functional groups like O-H stretch with the examination range of 3550-3200 have peaks at 3345.03 in API and 3345.14 in optimized formulation. Functional group N-H has a peak range of 3000-2800 has peaks at 2923.03 in API and 2918.85 in optimized formulation Table 4.
Figure 1:UV Spectrum for Vildagliptin at 245nm.
Figure 2:Standard curve of vildagliptin.
Figure 3:FTIR Spectrum of a) Vildagliptin b) Optimized blend.
| Characteristic peak | Literature values | Observed values | |
|---|---|---|---|
| Pure drug | Optimized formulation | ||
| O-H stretch | 3550-3200 | 3345.03 | 3345.14 |
| N-H | 3000-2800 | 2923.03 | 2918.85 |
Table 4: FTIR interpretation of API and optimized formulation.
| S.no | Formulations | B.D gm/ml | T.D gm/ml | C.I (%) | Angle of repose | Haunser ratio |
|---|---|---|---|---|---|---|
| 1 | F1 | 0.43 | 0.48 | 10.41 | 22.11 | 1.11 |
| 2 | F2 | 0.40 | 0.48 | 16.66 | 26.78 | 1.2 |
| 3 | F3 | 0.42 | 0.50 | 16.01 | 24.53 | 1.19 |
| 4 | F4 | 0.41 | 0.49 | 16.32 | 27.57 | 1.19 |
| 5 | F5 | 0.43 | 0.53 | 18.86 | 25.37 | 1.23 |
| 6 | F6 | 0.42 | 0.51 | 17.64 | 26.11 | 1.21 |
Table 5: Flow properties of formulations.
| Formula code | Hardness (Kg/cm2) | Weight variation (mg) | Friability (%) | Drug content (%) |
|---|---|---|---|---|
| F1 | 4.0 | 200 | 0.24 | 99.6 |
| F2 | 4.3 | 199 | 0.37 | 99.0 |
| F3 | 4.1 | 201 | 0.26 | 99.4 |
| F4 | 4.2 3 | 200 | 0.34 | 99. |
| F5 | 4.0 | 198 | 0.25 | 99.2 |
| F6 | 4.0 | 201 | 0.20 | 99.1 |
Table 6:Post formulation parameters of tablets.
| Time (hrs) | F1 | F2 | F3 | F4 | F5 | F6 |
|---|---|---|---|---|---|---|
| Dissolution medium 0.1N HCL | ||||||
| 0.5 | 4.9 | 10.9 | 7.1 | 19.4 | 28.5 | 10.1 |
| 1 | 10.1 | 20.27 | 16.9 | 29.4 | 36.8 | 38.9 |
| 2 | 21.4 | 29.16 | 28.7 | 38.8 | 49.8 | 50.1 |
| phosphate buffer 6.8pH | ||||||
| 3 | 37.5 | 32.8 | 41.8 | 49.6 | 67.4 | 72.8 |
| 4 | 49.7 | 45.41 | 56.1 | 59.1 | 84.7 | 100.1 |
| 6 | 67.2 | 56.71 | 73.2 | 78.6 | 98.3 | -- |
| 8 | 76.8 | 64.76 | 99.2 | 89.2 | -- | -- |
| 10 | 82.8 | 73.17 | -- | 100.1 | -- | -- |
| 12 | 96.2 | 86.13 | -- | -- | -- | -- |
Table 7:Drug release profile.
Pre-compression and post compression properties
Table 5,6
In-vitro dissolution studies
Dissolution parameters:
Disso Medium : 0.1N HCL (initial 2hrs),6.8pH phosphate buffer (up to 24hrs)
Type of apparatus : USP - II (paddle type)
RPM : 50
Bowl Volume : 900ml
Bowl Temperature : 37ºC± 0.5
Time : 2hrs
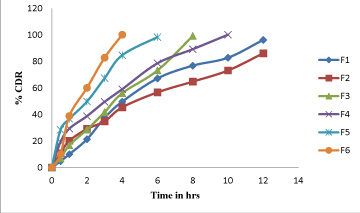
In-Vitro drug release studies for CR tablets: Table 7, Figure 4
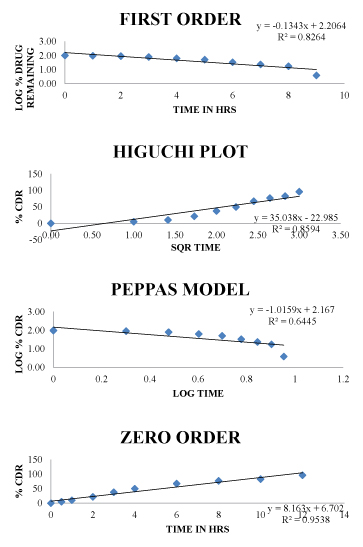
Kinetic studies for optimized formulation (F1) Figure 5
Summary and Conclusion
In the current work, an industrially vital project entitled “Formulation and Evaluation of Vildagliptin Controlled Release Tablets” was undertaken. The study was undertaken with an aim to formulate Vildagliptin as CR tablets. During this phase of exploration a variety of factors such as to affect the presentation of the CR was studied. The release kinetics, dissolution rate, process variables such as weight variation, hardness during compression are the same factors found serious during the expansion depends on the experimental finding. Initially Preformulation studies were done & results directed the further course of formulation. FTIR studies
Figure 4:Dissolution graph for vildagliptin tablets F1- F6.
Figure 5:Drug release kinetics of Optimized formulation (F1).
were conducted to known the compatibilities between the Vildagliptin API with polymers. Direct compression method used in present study. Mixed blends were evaluated for tests such as bulk density, Tapped density, Compressibility Index and Hausner ratio before being punched as tablets. Tablets were tested for weight variation, thickness and friability, in-vitro dissolution tests were performed and percentage drug release was calculated. Bulk density and tapped density of powder blend was evaluated. The results were range from.0.40-0.43.The angle of repose for the entire formulations blend was evaluated. The results range from.22.11-27.57.Compressibility index for the entire formulations blend was evaluated. The results range from.10.41-18.86.The Hausner`s ratio for the entire formulations blend was evaluated. The results range from 1.11-1.23. The prepared tablets in all the preparations obsessed good mechanical strength with enough hardness in between of 4.0 to 4.3 kg/sq cm. The weight variation in all the nine preparation was found to be 198 to 201 mg. The % drug content of all the tablets was found to be around 99 % which was within the acceptable limits. Dissolution tests were performed and percentage drug release was calculated. F1, F2 containing GUAR GUM in 10%, 15%. F3, F4 containing EC in 10%, 15%. F5, F6 containing XANTHAN GUM in 10%, 15%. From above all formulation F1 Formulation containing GUAR GUM in 10% gave highest vildagliptin drug release up to 12hrs with 96.2%. The formulations F2, F3, F4, F5 and F6 have shown release 86.13%, 99.2%, 100.1%, 98.3% and 100.1 % respectively. F1 formulation was shows highest drug release 96.2% up to 12h.The optimized F1 formulation has follows zero order, higuchi release kinetics.
Hauss DJ (2007) Oral Lipid-based Formulations. Advanced Drug Delivery Reviews 59: 667-676[ Ref ]
Siepmann J, Peppas NA (2001) Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Advanced Drug Delivery Reviews 48: 139-157[ Ref ]
Jannin V, Musakhanian J, Marchaud D (2008) Approaches for the development of solid and semi solid lipid based formulations. Advanced Drug Delivery Reviews 60: 734-746[ Ref ]
Robinson JR, Lee VHL (1987) Controlled Drug Delivery: Fundamentals and Applications. Marcel Dekker, New York.[ Ref ]
Rowe RC, Sheskey SJ, Owen CS (2006) Handbook of Pharmaceutical excipients, Pharmaceutical Press, United Kingdom.[ Ref ]
Hauss DJ (2007) Oral Lipid-based Formulations. Advanced Drug Delivery Reviews 59: 667-676[ Ref ]
Debjit Bhowmik (2014) Formulation and evaluation of vildagliptin sustained release tablets. Research gate 2: 1293-1299.[ Ref ]
Priyanka Shrestha, Kumar K. Shiva M, Selim Reza Md. (2014) Design and Development of Immediate and Sustained Release Tablets of Vildagliptin. Research gate 5: 975-985.[ Ref ]
https://www.drugbank.ca/drugs/DB04876[ Ref ]
Tripathi KD (2003) Essentials of Medical Pharmacology. Jaypee Publishers, New Delhi.[ Ref ]
Raymond CR, Paul JS (2009) Hand book of pharmaceutical excipients.[ Ref ]