Journal Name: Journal of Drug Development and Delivery
Article Type: Research
Received date: 11 April 2019
Accepted date: 30 April 2019
Published date: 07 May 2019
Citation: Fathima S, Sridhar R (2019) Formulation and Evaluation of Clonazepam Oro Dispersible Tablets. J Drug Dev Del Vol: 2, Issu: 1 (19-24).
Copyright: © 2019 Fathima S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The main objective of the present research work is to develop stable formulation of oral disintegrating tablets (ODT) of anticonvulsant drug. In present work oral disintegrating tablets of Clonazepam drug were prepared by direct compression method. Six different formulations were studied to develop optimized robust formulation, different formulations were prepared by varying the concentration and combination of super disintegrants like Croscarmellose Sodium (CCS), Crospovidone (CP) and Sodium starch glycolate (SSG). Prepared six formulations were evaluated for weight variation, hardness, content uniformity, friability, Disintegration time, wetting time and invitro dissolution studies. Optimized formulation dissolution data is fitted for different kinetic modules to better understand drug release pattern. Stability studies shows optimized formulation was stable at different conditions as per ICH guidelines.
Keywords:Oral disintegrating tablets (ODT), Clonazepam, Crospovidone, Croscarmellose sodium, Sodium starch glycolate, Anticonvulsant drug.
Abstract
The main objective of the present research work is to develop stable formulation of oral disintegrating tablets (ODT) of anticonvulsant drug. In present work oral disintegrating tablets of Clonazepam drug were prepared by direct compression method. Six different formulations were studied to develop optimized robust formulation, different formulations were prepared by varying the concentration and combination of super disintegrants like Croscarmellose Sodium (CCS), Crospovidone (CP) and Sodium starch glycolate (SSG). Prepared six formulations were evaluated for weight variation, hardness, content uniformity, friability, Disintegration time, wetting time and invitro dissolution studies. Optimized formulation dissolution data is fitted for different kinetic modules to better understand drug release pattern. Stability studies shows optimized formulation was stable at different conditions as per ICH guidelines.
Keywords:Oral disintegrating tablets (ODT), Clonazepam, Crospovidone, Croscarmellose sodium, Sodium starch glycolate, Anticonvulsant drug.
Introduction
Oral administration of drugs is preferred due to its ease of swallowing, distress escaping, adaptability, most considerably and patient compliance.
Mechanism of ODT drugs
Generally, ODTs are formulated to disperse rapidly in the mouth, enabling medication to be swallowed without water, thereby enhancing convenience & compliance across a broad range of indications and patient types, including the young, elderly, and active patients. Following dispersion, the formulations are swallowed, and the drug is absorbed in the same way as conventional solid-oral dosage forms.
Materials and Methods
Materials
Clonazepam, Crospovidone, Cros carmellose sodium and Sodium starch glycolate were gifted sample from Chandra labs, Hyderabad. Other chemicals like MCC, Mg stearate and Talc were analytical grade.
Methods
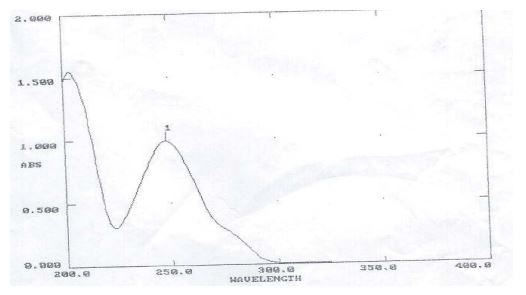
Calibraton curve of Clonazepam: Weighed 100 mg of Clonazepam and shifted into 100 ml of volumetric flask & dissolved in less amount of water and diluted with phosphate buffer of pH 6.8 up to the mark to give stock solution 1 mg/ml. 1 ml was pick out from stock solution in another volumetric flask and diluted up to 10 ml to give a standard solution 100 µg/ml. Further dilutions were made from 10-50 µg/ml using phosphate buffer pH 6.8 and absorbance were measured at 254 nm (figure no.1 spectrum of clonazepam drug) Figure 1.
Figure 1: Spectrum of Clonazepam in 6.8 PH buffer.
| Ingredients (mg) | C1 | C2 | C3 | C4 | C5 | C6 |
|---|---|---|---|---|---|---|
| Clonazepam | 1mg | 1mg | 1mg | 1mg | 1mg | 1mg |
| Crospovidone + croscarmellose sodium | 7.5 | 15 | ------ | ------ | ------ | ------ |
| Sodium starch glycolate+ Crospovidone | ------ | ------ | 7.5 | 15 | ------ | ------ |
| Sodium starch glycolate+ croscarmellose sodium | ----- | ----- | ----- | ----- | 7.5 | 15 |
| MCC | Qs | qs | qs | qs | Qs | qs |
| Magnesium stearate | 3 | 3 | 3 | 3 | 3 | 3 |
| Talc | 3 | 3 | 3 | 3 | 3 | 3 |
| Total tablet weight | 150mg | 150mg | 150mg | 150mg | 150mg | 150mg |
Table 1: Formulations table for Clonazepam ODT.
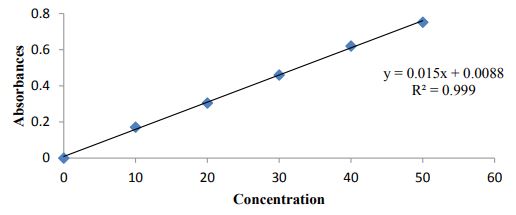
| Concentration (µg/mL) | Absorbance |
|---|---|
| 0 | 0 |
| 10 | 0.171 |
| 20 | 0.305 |
| 30 | 0.3460 |
| 40 | 0.620 |
| 50 | 0.752 |
Table 2: Standard calibration curve of Clonazepam in phosphate buffer.
Figure 2:Standard curve of Clonazepam in 6.8 PH buffer.
Drug and Excipients compatibility studies: The selection of excipients was based on the prior experience or literature survey; excipients listed by the innovator in the package insert (Micardis) tablets. Compatibility was observed of these excipients with Clonazepam. In the present study, the potassium bromide disc (pellet) method was employed.
Preparation of Clonazepam ODT’s3: The main aim of the present study was to formulate different batches using three various superdisintegrants and other ingredients in varying concentrations. So, different batches of formulations were planned accordingly. According to that C1, C2 formulations (with Crospovidone + croscarmellose sodium - 5%, 10%), C3, C4 formulations (with Sodium starch glycolate+ Crospovidone - 5%, 10%),C5,C6 formulations (with Sodium starch glycolate + croscarmellose sodium - 5%,10%) prepared by direct compression method (Table 1).
Results
Calibration curve of Clonazepam drug in phosphate buffer
Table 2 and Figure 2
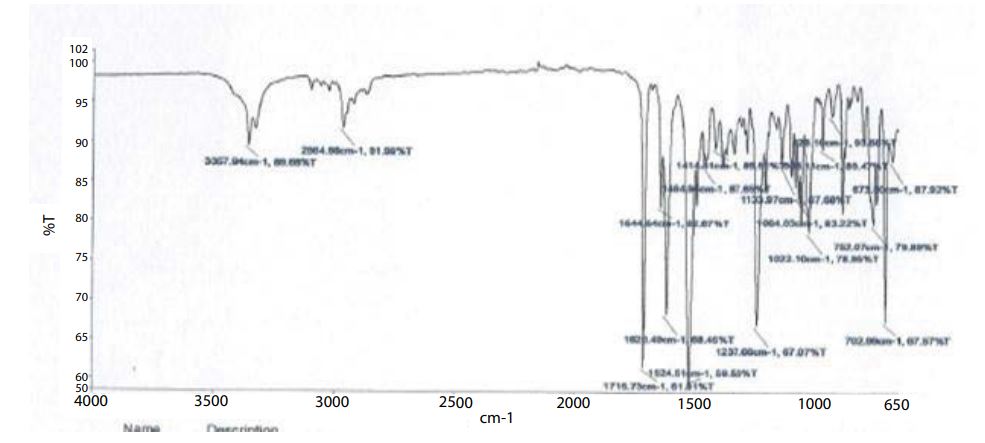
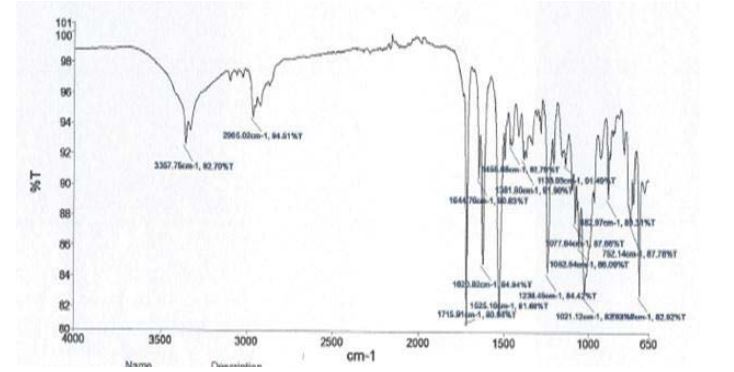
Drug and excipient compatibility studies (FTIR)
Figures 3 and 4
Evaluation of Prepared Clonazepam Oral disintegrating tablets
Table 3
In -vitro drug release study
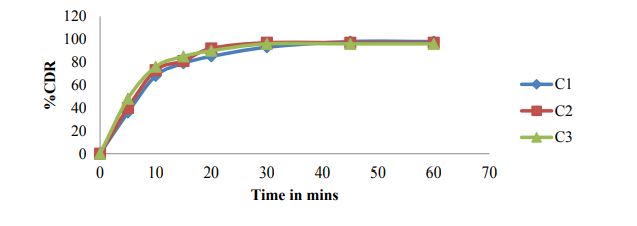
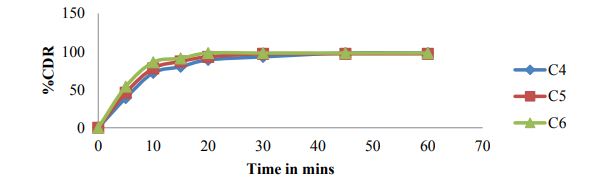
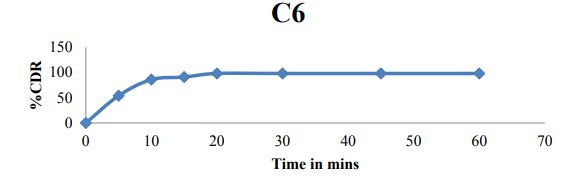
Paddle method Dissolution data of Matrix tablets formulations of Clonazepam by Paddle method (USP II) are reported in table 4 and cumulative % drug release were shown in figures 5 to 7.
Kinetic studies for optimized formulation (C6)
Table 5 and Figures 8 to 11.
Stability studies
Table 6
Summary and Conclusion
In the current work, immediate release tablets of Clonazepam were prepared by direct compression technique. The designed formulations were subjected into weight of variation, drug uniformity content, hardness & friability, and post formulation studies like as ratio of water absorption, wetting time, dissolution, drug excipients interaction and short-term stability studies.
• IR-spectroscopic studies indicated that there are no drug–excipients interactions.
• By the direct compression the designed tablets were punched without any chipping, capping and sticking.
• The prepared tablets of the hardness were found to be in b/w of 1.1 to 1.8kg/ cm².
• The values of friability were found to be in b/w of 0.65 to 0.72%.
• Disintegration time was found to be in b/w of 1-3min.
• The average weight & content of drug prepared tablets indicate weight and uniformity of drug content within the batches prepared.
• Maximum drug release was found to be with formulation C6 (98%).
Figure 3:FTIR of Clonazepam pure drug.
Figure 4: FTIR of Clonazepam optimized formulation.
| Formulation code | Weight variation | Hardness( kg/cm²) | Friability(%) | Thickness(mm) | Content uniformity | Disintegration Time (min) | Wetting time in5 (min) |
|---|---|---|---|---|---|---|---|
| C1 | 149 | 1.6 | 0.72 | 1.4 | 99.28 | 2 | 1.20 |
| C2 | 151 | 1.5 | 0.68 | 1.6 | 97.16 | 2.4 | 1.35 |
| C3 | 150 | 1.8 | 0.69 | 1.7 | 101.1 | 2.4 | 2.15 |
| C4 | 148 | 1.7 | 0.66 | 1.5 | 97.68 | 2.2 | 2.08 |
| C5 | 148 | 1.8 | 0.68 | 1.6 | 98.19 | 2.3 | 1.45 |
| C6 | 150 | 1.1 | 0.65 | 1.2 | 99.41 | 1.4 | 1.15 |
Table 3: Evaluation tests of different formulations.
| Time in min | C1 | C2 | C3 | C4 | C5 | C6 |
|---|---|---|---|---|---|---|
| 5 | 36 | 40 | 48 | 39 | 46 | 54 |
| 10 | 68 | 73 | 76 | 72 | 78 | 86 |
| 15 | 79 | 81 | 85 | 80 | 87 | 91 |
| 20 | 85 | 92 | 90 | 89 | 93 | 98 |
| 30 | 93 | 97 | 96 | 93 | 97 | 98 |
| 45 | 98 | 97 | 96 | 98 | 97 | 98 |
| 60 | 98 | 97 | 96 | 98 | 97 | 98 |
Table 4: In-vitro dissolution data of prepared tablets.
Figure 5: Cumulative % drug released for formulations C1-C3.
Figure 6: Cumulative % drug released for formulations C4-C6.
Figure 7: Cumulative % drug release of C6 formulation.
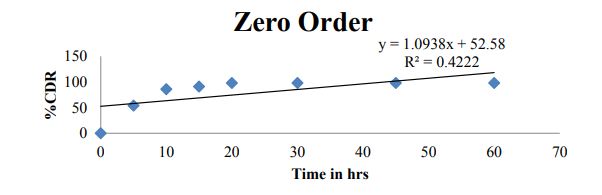
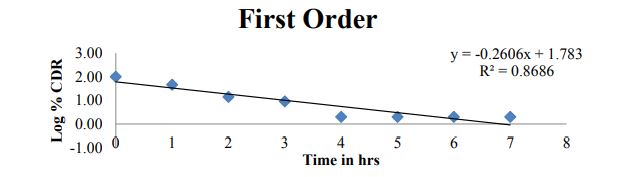
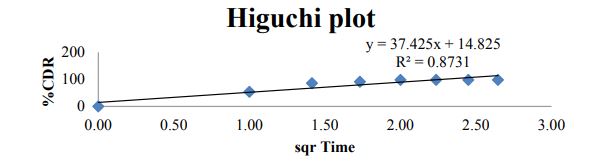
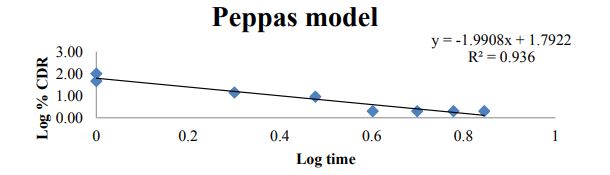
| ZERO | HIGUCHI | PEPPAS | FIRST | |
|---|---|---|---|---|
| % CDR Vs T | %CDR Vs √T | Log C Vs Log T | Log % Remain Vs T | |
| Slope | 1.093 | 37.42 | -1.990 | -0.260 |
| Intercept | 52.58 | 14.82 | 1.792 | 1.783 |
| R 2 | 0.422 | 0.873 | 0.936 | 0.868 |
Table 5: Drug release kinetics profile.
Figure 8: Zero order plot for optimized formulation C6.
Figure 9: First order plot for optimized formulation C6.
Figure 10: Higuchi plot for optimized formulation C6.
Figure 11: Peppas plot for optimized formulation C6.
| S.NO | Parameters | Initial | 1 month | 2 month | 3 month | Limits as per specification |
|---|---|---|---|---|---|---|
| 1 | 400C/75% RH % Release | 98 | 98.52 | 97.79 | 96.56 | Not less than 85 % |
| 2 | 400C/75% RH Assay Value | 98 | 97.96 | 96.22 | 96.00 | Not less than 90 % Not more than 110 % |
Table 6: Results of stability studies of optimized formulation C-6.
Stability studies
There was no significant change in physical and chemical properties of the tablets of formulation C6 after 3 Months, parameters like % drug release and assay values at various conditions (at 40°C/ 75% RH) as per ICH guidelines quantified at various time intervals.
Leon Lachmann, Herbert AL, Joseph LK (2014) The theory and practice of Industrial Pharmacy.[ Ref ]
Ansel`s Pharmaceutical dosage forms & drug delivery systems (2013) Valabh Prakashan.[ Ref ]
Debjit B, Chiranjib B, Krishnakanth, Pankaj, Margret Chandira R (2009) Fast Dissolving Tablet: An Overview. Journal of Chemical and Pharmaceutical Research 1: 163-177.[ Ref ]
Susijit Sahoo, Mishra B, Biswal P, Omprakash Panda, Satosh Kumar M, et al. (2010) Fast Dissolving Tablet: As A Potential Drug Delivery System. Drug Invention Today 4: 130-133.[ Ref ]
A Gupta, AK Mishra, V Gupta, P Bansal, R Singh, et al. (2010) Recent Trends of Fast Dissolving Tablet - An Overview of Formulation Technology. International Journal of Pharmaceutical & Biological Archives 1: 1-10.[ Ref ]
Reddy LH (2002) Fast dissolving drug delivery systems: A review of the literature. International journal of pharmaceutical Sciences 5: 331-336. [ Ref ]
Metker V, Kumar A (2011) Formulation and evaluation of orodispersible tablets of Lornoxicam. International journal of drug development and research 3: 281-285.[ Ref ]
Khalid Abed, Ahmed A Hussein (2010) Formulation and Optimization of orodispersible tablets of Diazepam. AAPS pharmsciTech 11: 356-361.[ Ref ]
Sandhyarani G, Sarangapani M (2016) Formulation and evaluation of orodispersible tablets of Domperidone. IOSR journal of pharmacy 6: 39-47.[ Ref ]
Rajnikant MS, Narendra PC, Digesh DS (2013) Formulation and evaluation of fast dissolving tablets of ondonsetron by solid dispersion in superdisintegrants. Indian journal of pharmaceutical education and research 47: 49-55.[ Ref ]
Nagendrakumar D, Raju SA (2009) Fast dissolving tablets of fexofenadine Hcl by effervescent method. Indian journal of pharmaceutical sciences 71: 116-119.[ Ref ]
Shirsand SB, Sarasija Suresh (2010) Formulation design and optimization of fast disintegrationg lorazepam tablets by effervescent method. Indian journal of pharmaceutical sciences 72: 431-436.[ Ref ]