Journal Name: Journal of Drug Development and Delivery
Article Type: Research
Received date: 12 June 2019
Accepted date: 08 July 2019
Published date: 15 July 2019
Citation: Unnisa R, Sridhar Babu R, Syed Rahmath A (2019) Formulation and In-vitro Evaluation of Bosentan Sustained Release Tablets. J Drug Dev Del Vol: 2, Issu: 2 (01-07).
Copyright: © 2019 Unnisa R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
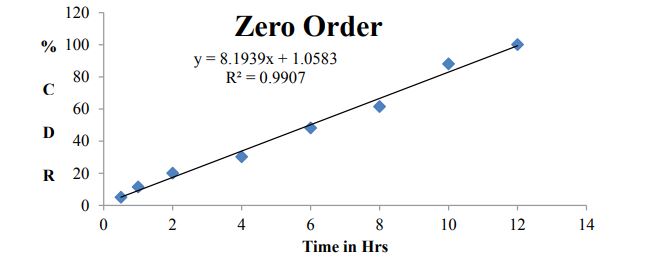
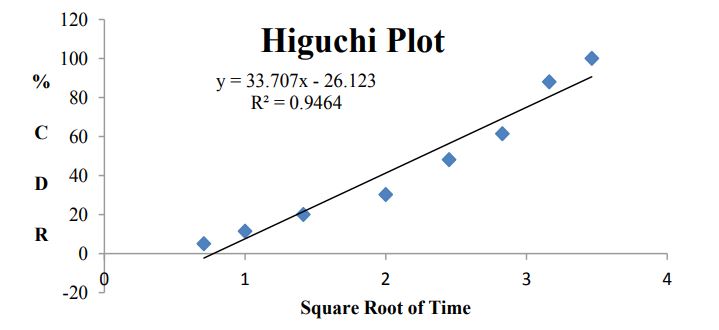
Model drug used in present investigation has relatively short plasma half-life and low bioavailability (~50%). Two to three times administration was required for larger doses, this can decrease the patient compliance. Sustained release formulation maintains plasma level for 10-12hrs and it might be sufficient for daily dosing of bosentan. Main objective of the present investigation was to develop an oral sustained release bosentan formulation by using different polymers in combination with Hydroxy propyl methyl cellulose (HPMC). Tablets were prepared by direct compression method. In vitro dissolution studies were carried out for all nine formulations in simulated gastric fluid using USP type II dissolution apparatus for 10-12hrs. Dissolution data was analyzed using different kinetic models. B7 formulation with combination of polymers HPMC and ethyl cellulose (EC) shows sustained drug release upto 12hrs. Kinetic modeling of In-vitro drug release study of optimized formulation indicates dissolution follows zero order, fitting dissolution data to higuchi model revealed super case II transport.
Keywords:Hydroxy propyl methyl cellulose, Sustained release, Simulated gastric fluid, Kinetic models, Ethyl cellulose.
Abstract
Model drug used in present investigation has relatively short plasma half-life and low bioavailability (~50%). Two to three times administration was required for larger doses, this can decrease the patient compliance. Sustained release formulation maintains plasma level for 10-12hrs and it might be sufficient for daily dosing of bosentan. Main objective of the present investigation was to develop an oral sustained release bosentan formulation by using different polymers in combination with Hydroxy propyl methyl cellulose (HPMC). Tablets were prepared by direct compression method. In vitro dissolution studies were carried out for all nine formulations in simulated gastric fluid using USP type II dissolution apparatus for 10-12hrs. Dissolution data was analyzed using different kinetic models. B7 formulation with combination of polymers HPMC and ethyl cellulose (EC) shows sustained drug release upto 12hrs. Kinetic modeling of In-vitro drug release study of optimized formulation indicates dissolution follows zero order, fitting dissolution data to higuchi model revealed super case II transport.
Keywords:Hydroxy propyl methyl cellulose, Sustained release, Simulated gastric fluid, Kinetic models, Ethyl cellulose.
Introduction
Most conventional oral drug products, such as tablets and capsules, are formulated to release and complete systemic drug absorption immediately after oral administration of prodrug. Such immediate release tablets result in relatively rapid drug absorption and onset of accompanying pharmacodynamic effects. In recent years, various modified-release drug products have been developed to control the release rate of the drug and/or the time for drug release [1].
The term modified-release drug product is used to describe products that alter the timing and/or the rate of release of the drug substance. A modified-release dosage form is defined “as one for which the drugrelease characteristics of time course and/or location are chosen to accomplish therapeutic or convenience objectives not offered by conventional dosage forms such as solutions or promptly dissolving dosage forms as presently recognized” [2-5].
Several types of modified-release drug products are recognized
Extended-release drug products: A dosage form that allows at least a twofold reduction in dosage frequency as compared to that drug presented as an immediate-release (conventional) dosage form. Examples of prolonged-release dosage forms include controlled-release, sustained-release, and long-acting drug products [6].
The term controlled-release drug product was previously used to describe various types of oral extended-release-rate dosage forms, including sustained-release, sustained-action, prolongedaction, long-action, slow-release, and programmed drug delivery [7].
Sustained drug release(SR): The goal of sustained –release dosage form is to maintain therapeutic blood or tissue levels of the drug for an extended period. Commonly, the release rate of drug is not altered and does not result in sustained delivery once drug release has begun [8-10].
Classification of oral sustained release systems
A.Diffusion Sustained Systems: Reservoir devices and Matrix devices
B. Dissolution controlled systems: Matrix systems and Encapsulation systems
C. Diffusion and Dissolution Controlled System: In a bio-erodible matrix, the drug is homogenously dispersed in a matrix and it is released either by swelling controlled mechanism or by hydrolysis or by enzymatic attack [11-14].
Materials and Methods
Materials
Bosentan drug was gifted sample from Chandra labs, Hyderabad. Polymers Hydroxy propyl methyl cellulose (HPMC), Ethyl cellulose (EC), Xanthan gum, Acacia were purchased from MYL chemicals, Mumbai. Micro crystalline cellulose (MCC), Magnesium stearate and Talc were laboratory standards.
Methodology
Pre-formulation studies: 4.2.1.1 Analytical study: Drug description, solubility studies in different solvents, determination of melting point and scanning of drug using UV Visible spectrophotometer.
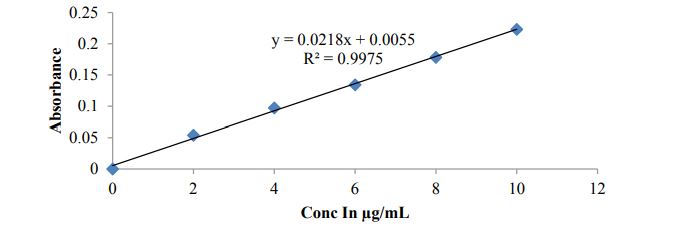
Construction of standard graph of Bosentan in 0.1N HCL: Bosentan drug dissolved in 0.1N HCL and preparae concentrations from 2-10 μg/ml and absorbance were noted at 204nm using UV Visible spectrophotometer. Linearity graph was plotted with these observations against concentrations
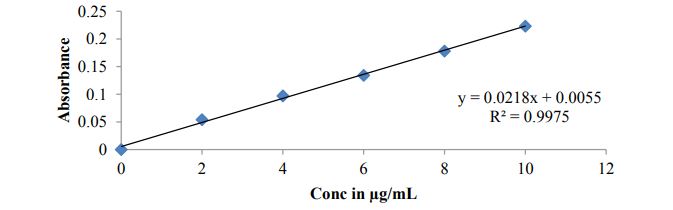
Construction of linearity curve of Bosentan in pH 6.8 Phosphate buffer: 2-10 μg/ml concentrations of drug solutions were prepared using pH 6.8 Phosphate buffer as solvent and note the absorbance for the same concentrations using UV Visible spectrophotometer at lamda max 204nm. Concentrations were plotted on X-axis and noted absorbance for respective concentrations were plotted on Y-axis to achieve calibration curve.
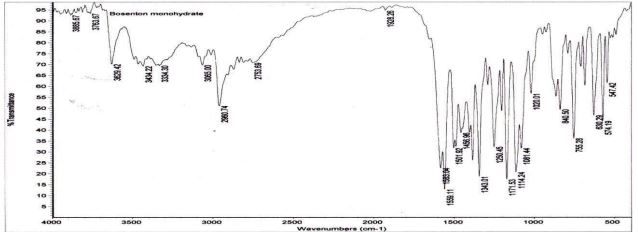
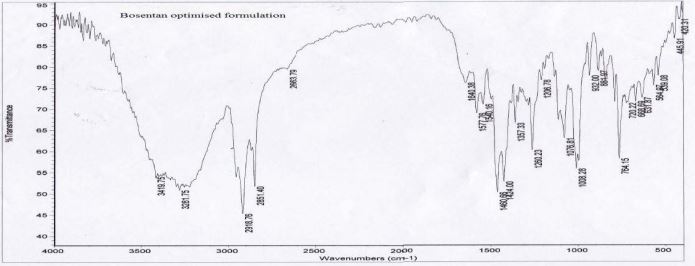
Drug – excipient compatibility study: The IR spectrum of the physical mixture was compared with that of the pure drug to check any possible drug-excipient interaction.
Formulation of sustained release tablets: 4.2.3.1 Precompression parameters: Mixed blend was evaluated for flow properties like Bulk density, Tapped density, Angle of repose, Hausner ratio and compressibility index.
Preparation of Bosentan SR tablets: Bosentan SR tablets were prepared using direct compression method shown in table 1.
Evaluation of SR tablets: Prepared tablets were evaluated for Physical appearance, Size & shape, weight variation test, Friability, Hardness, Drug content and In-vitro dissolution studies.
In-vitro drug release studies: Bosentan SR tablets dissolution studies were conducted in USP type II dissolution apparatus, first two hours in 0.1N HCL and remaining time for 6.8pH phosphate buffer to simulate in vivo gastric conditions.
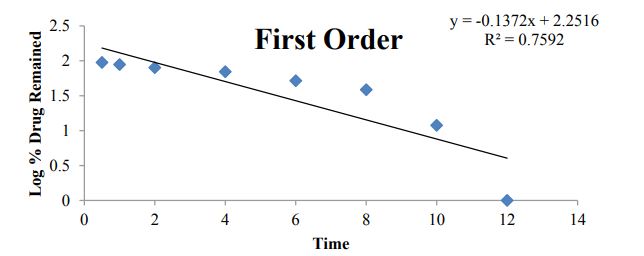
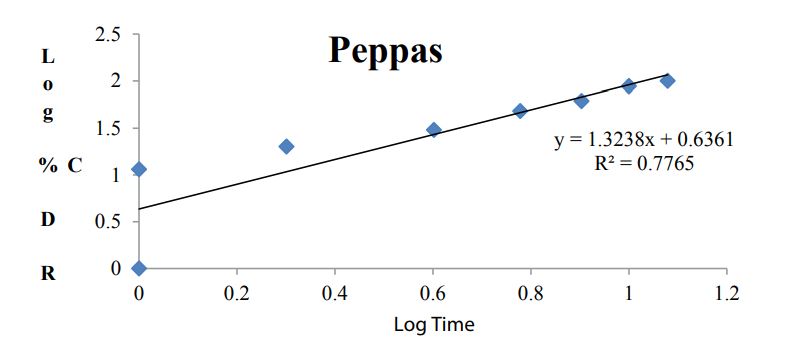
Kinetic analysis of dissolution data: To analyze the in vitro release data various kinetic models were used to describe the release kinetics: Zero order kinetics, First order kinetics, Higuchi plot and Kosmeyar Peppas model explained in table 2.
Results and Discussion
Pre-formulation studies
Drug description: Observations were found as per specifications only showed in table 3.
Solubility: Bosentan drug solubility increases with increasing the pH of the solvent and poorly soluble in water discussed in table 4.
Melting point: Bosentan (API) melting point was observed at 1380c and within the limits only.
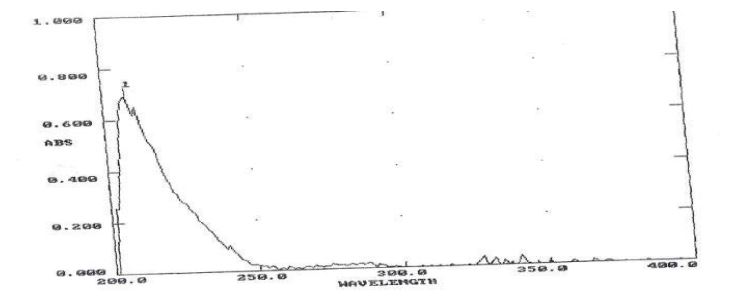
Scanning of API: Figure 1
Standard calibration curve of Bosentan
Figure 2 and Figure 3
Drug-excipient compatibility study (FTIR)
Figure 4 and Figure 5
Pre compression parameters of formulations
Table 5
Post compression parameters for formulations
Table 6
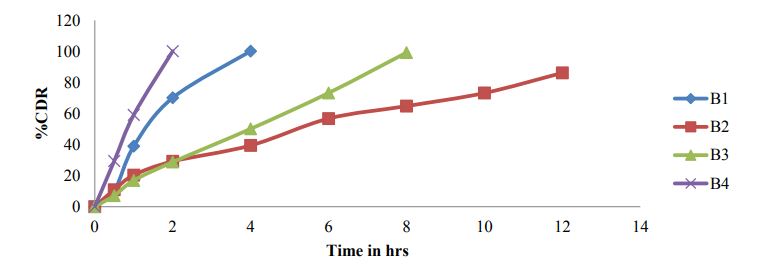
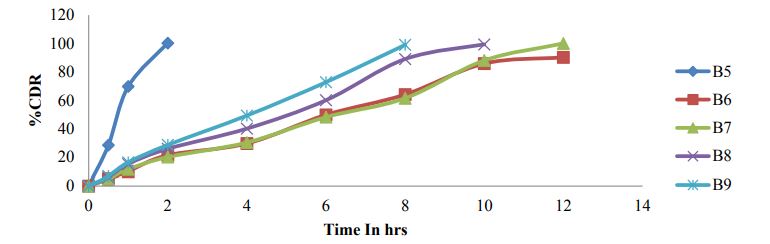
In-vitro dissolution studies
Figure 6 and Figure 7
Kinetic studies for optimized formulation (B7)
Table 7 and Figures 8-11
| Ingredients (%) | B1 | B2 | B3 | B4 | B5 | B6 | B7 | B8 | B9 |
|---|---|---|---|---|---|---|---|---|---|
| Bosentan (mg) | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 |
| HPMC | 10% | 20% | 30% | 10% | 20% | 30% | 10% | 20% | 30% |
| Xanthan gum | 10% | 20% | 30% | ||||||
| Acacia | 10% | 20% | 30% | ||||||
| Ethyl cellulose | 10% | 20% | 30% | ||||||
| MCC | Qs | Qs | Qs | Qs | Qs | Qs | Qs | Qs | Qs |
| Magnesium Stearate | 2% | 2% | 2% | 2% | 2% | 2% | 2% | 2% | 2% |
| Talc | 2% | 2% | 2% | 2% | 2% | 2% | 2% | 2% | 2% |
| Total wt (mg) | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 |
Table 1: Formulation table for Bosentan SR tablets.
| Diffusion exponent (n) | Overall solute diffusion mechanism |
|---|---|
| 0.45 | Fickian diffusion |
| 0.45 < n < 0.89 | Anomalous (non-Fickian) diffusion |
| 0.89 | Case-II transport |
| n > 0.89 | Super case-II transport |
Table 2: Diffusion Exponent and Solute Release Mechanism for Cylindrical Shape.
Figure 1: UV Spectrum for Bosentan observed at 204nm.
Figure 2: Calibration curve of Bosentan in 0.1N HCL.
Figure 3: Calibration curve of Bosentan in pH 6.8 Phosphate buffer.
Figure 4: FTIR of Bosentan pure drug.
Figure 5: FTIR of Bosentan optimized formulation.
| Test | Description |
|---|---|
| Colour | White to off white powder |
| Odour | Free of odour |
Table 3: Table showing the description of Bosentan (API).
| Solvents | Solubility |
|---|---|
| Water | Poorly soluble |
| pH6.8 Phosphate buffer | Soluble |
| Methanol | Soluble |
| Chloroform | Soluble |
Table 4: Table showing the Solubility of Bosentan (API) in various solvents.
| S.no | Formulations | Bulk Density | Tapped Density | Compressibility | Angle of repose | Haunser ratio |
|---|---|---|---|---|---|---|
| (gm/ml) | (gm/ml) | index (%) | (ᶱ) | |||
| 1 | B1 | 0.44 | 0.52 | 15.38 | 25.10 | 1.18 |
| 2 | B2 | 0.42 | 0.49 | 14.29 | 26.79 | 1.17 |
| 3 | B3 | 0.43 | 0.51 | 15.69 | 24.54 | 1.19 |
| 4 | B4 | 0.41 | 0.48 | 14.58 | 27.56 | 1.17 |
| 5 | B5 | 0.44 | 0.52 | 15.38 | 25.38 | 1.18 |
| 6 | B6 | 0.43 | 0.50 | 14.00 | 28.10 | 1.16 |
| 7 | B7 | 0.48 | 0.56 | 14.29 | 25.49 | 1.17 |
| 8 | B8 | 0.47 | 0.54 | 12.96 | 24.57 | 1.15 |
| 9 | B9 | 0.45 | 0.53 | 15.09 | 26.45 | 1.18 |
Table 5: Precompression parameters of formulation blend.
| Formula code | Hardness(Kg/cm2) | Weight variation (mg) | Friability (%) | Drug content (%) |
|---|---|---|---|---|
| B1 | 5.2 | 200 | 0.26 | 99.6 |
| B2 | 5.4 | 199 | 0.35 | 99.0 |
| B3 | 5.0 | 200 | 0.28 | 99.4 |
| B4 | 5.9 | 202 | 0.33 | 99.3 |
| B5 | 5.8 | 198 | 0.28 | 99.2 |
| B6 | 5.0 | 200 | 0.5 | 99.5 |
| B7 | 5.2 | 201 | 0.45 | 99.8 |
| B8 | 5.1 | 199 | 0.35 | 99.1 |
| B9 | 5.0 | 199 | 0.35 | 99.4 |
Table 6: Post formulation parameters of tablets.
Figure 6: Dissolution profile of Bosentan formulations B1-B4.
Figure 7: Dissolution profile of Bosentan SR formulations B5-B9.
Figure 8: Zero order plot for B7 dissolution data.
Figure 9: First order plot for B7 dissolution data.
Figure 10: Higuchi modeling for B7 formulation data.
Figure 11: Higuchi modeling for B7 formulation data.
Summary and Conclusion
Present study was undertaken with an aim to formulate ........
**** Page no-6 ****Patient was suffering from liver cirrhosis secondary to hepatitis which is irreversible state. Her chief complaints were abdominal pain, abdominal distension and pedal edema. USG report showed end stage liver cirrhosis, coarse and necrotic liver. The risk behind this severe suffering was the hepatitis in the past left untreated which appeared as a complication, patient was on palliative care to provide patient with relief from the symptoms, pain, and stress of a serious illness, such as cirrhosis. The goal of palliative care is to improve quality of life for both the patient and the patient’s family and it is appropriate at any stage and for any type of cirrhosis.[11] Desired outcomes of such patient should be to minimize continued liver damage, optimize nutrition, maximize hepatic circulation, minimize and prevent respiratory complications.[9]Lifestyle interventions are beneficial including smoking cessation and stop drinking alcohol, limit salt in diet, get vaccinated for hepatitis A & B, and pneumococcal pneumonia. Liver damage from cirrhosis cannot be reversed, but the treatment could stop or delay further progression and reduce complications. [12]
Swarbrick J, Boylan JC (1991) Encyclopedia of Pharmaceutical Technology. Marcel Dekker INC, New York.[ Ref ]
Trapani G, Franco M, Latrofa A, Tullio C, Provenzano MR, et al. (2001) Dissolution properties and anticonvulsant activity of Phenytoin polyethylene glycol 6000 and poly vinyl pyrrolidone K-30 solid dispersions. Int J Pharm 225: 63-73.[ Ref ]
Chiou WL, Riegelman S (1971) Pharmaceutical applications of solid dispersion systems. J Pharm Sci 60: 1281-1302. [ Ref ]
Corrigan OI, Stanley T (1985) Mechanisms of dissolution of fast release solid dispersions. Drug Dev Ind Pharm 11: 697-724.[ Ref ]
Serajuddin ATM (1999) Solid dispersion of poorly water soluble drugs: early promises, subsequent problems and recent break through. J Pharm Sci 88: 1058-1066.[ Ref ]
Gaurav Agarwal, Shilpi Agarwal, Karar PK, Shagun G (2017) Oral sustained release tablets: an overview with a special emphasis on Matrix tablet. American journal of advanced drug delivery 5: 64-76.[ Ref ]
Ashwini R, Bhalekar MR, Kolhe VJ, Kenjale YD (2009) Formulation and optimization of sustained release tablets of Venlafaxine resinates using response surface morphology. Indian journal of pharmaceutical sciences 71: 387-394.[ Ref ]
Sumathi S, Ray AR (2002) Release behavior of drugs from tamarind seed polysaccharide tablets. J Pharm Sci 5: 12-18. [ Ref ]
Raymond CR, Paul JS (2001) Hand book of pharmaceutical excipients. Pharmaceutical Press, United Kingdom.[ Ref ]
Kaushic D, Dureja H, Saini T (2004) Mouth dissolving tablet: A review. Indian Drugs 41: 187-193.[ Ref ]
Yeola B, Pisal S, Paradkar A, Mahadik K (2000) New drug delivery system for elderly. Indian Drugs 13: 312-318. [ Ref ]
Sastry S, Nyshdham J, Fix J (2000) Recent technological advances in oral drug delivery: A review. AAPS Pharm Sci Tech 13: 138-144.[ Ref ]
Prajapati ST, Gohel MC, Patel LD (2007) Studies to enhance dissolution properties of carbamazepine. Indian J Pharm Sci 69: 427-430.[ Ref ]
Quian F, Huang J, Hussain MA (2010) Stability Consideration and Practical Challenges in Amorphous Solid Dispersion Development. J Pharm Sci 99: 2941-2947.[ Ref ]