Journal Name: Journal of Drug Development and Delivery
Article Type: Research
Received date: 12 June 2019
Accepted date: 17 July 2019
Published date: 24 July 2019
Citation: Begum RU, Sridhar Babu R, Kishor DV, Begum NU (2019) Formulation and In-vitro Evaluation of Ciclopirox Ethosomal Gel. J Drug Dev Del Vol: 2, Issu: 2 (08-16).
Copyright: © 2019 Begum RU. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Ethosomal systems were lipid vesicular carriers which contain high percentage of ethanol and ethosomal gels were prepared in present study using ciclopirox as model drug. EF1-EF12 different formulations were prepared by varying concentration of ethanol from 20-50% and soya lecithin from 2-4%. Ethosome formulations were characterized for compatibility, drug entrapment efficiency, drug content, invitro drug release studies, surface morphology, vesicle size and size distribution. Ethosomal gel was prepared for optimized ethosomal formulation EF7 by incorporated into 1-2% carbopol gel. Ethosomal gels were characterized for pH, viscosity and spreadability. Optimized ethosomal formulation’s invitro drug release pattern was studied for different release kinetic models.
Keywords
Ethosomal system, Lipid vesicular carriers, Surface morphology, Entrapment efficiency.
Abstract
Ethosomal systems were lipid vesicular carriers which contain high percentage of ethanol and ethosomal gels were prepared in present study using ciclopirox as model drug. EF1-EF12 different formulations were prepared by varying concentration of ethanol from 20-50% and soya lecithin from 2-4%. Ethosome formulations were characterized for compatibility, drug entrapment efficiency, drug content, invitro drug release studies, surface morphology, vesicle size and size distribution. Ethosomal gel was prepared for optimized ethosomal formulation EF7 by incorporated into 1-2% carbopol gel. Ethosomal gels were characterized for pH, viscosity and spreadability. Optimized ethosomal formulation’s invitro drug release pattern was studied for different release kinetic models.
Keywords
Ethosomal system, Lipid vesicular carriers, Surface morphology, Entrapment efficiency.
Introduction
Transdermal drug delivery systems are externally applied medicaments in the form of gels that deliver drugs for systemic effects at a predestined and controlled rate [1,2]. Through a diffusion process, the drug enters the bloodstream directly through the skin. Since there is high concentration in ethosomal gel and low concentration in the blood, the drug will keep diffusing into the blood for an extended period of time, maintaining the constant concentration of drug in the blood flow [3-7].
Transdermal delivery is an important delivery route that delivers precise amount of drug through the skin for systemic action [8]. Throughout the past two decades, the transdermal drug delivery has become a proven technology holding the promise that new compound could be delivered in a safe and convenient way through the skin.
Ethosomes
Vesicles would also allow to control the release rate of drug over an extended time, keeping the drug shielded from immune response or other removal systems and would be able to release just the right amount of drug and keep that concentration constant for longer periods of time (Figure 1). Ethosomal vesicles have more benefits in comparison to other transdermal delivery systems. Ciclopirox is an antifungal agent used in present study as model drug.
Materials and Methods
Materials
Drug ciclopirox was gifted by Chandra labs, Hyderabad. Soya lecithin and cholesterol were purchased from standard chemicals and reagents, Hyderabad. Carbopol, PG (propylene glycol), ethanol, TEA (triethanolamine) and water were laboratory standards. Phosphate buffer 7.4pH was prepared as per pharmacopeial guidelines.
Figure 1: Structure of ethosomes.
Methodology
Preparation of ethosomal vesicles: Ciclopirox ethosomal formulations were prepared (shown in table no.1) by ‘Cold method’, drug concentration was fixed to 8% w/v, vesicle forming agent soya lecithin concentration in formulation was varied from 2-4% and ethanol concentration was adjusted from 20-50%. In this method phospholipids and lipid materials were dissolved in ethanol at room temperature by magnetic stirring process with addition of penetration enhancer PG continuously. Add drug with constant stirring followed by heating the above mixture at 30oC in water bath. In separate vessel heat water at 30oC and mix both the phases together, stir for 5min. Vesicle size of ethosomes can be decreased by extending the sonication process [9-11]. Final formulation stored under refrigeration.
Preparation of ethosomal gel: Dissolve TEA in water under stirring and add carbopol slowly, allow hydrating under stirring for 20-30min [12]. Ethosomes equivalent to 8% of Ciclopirox were collected and dispersed in the hydrated polymer slurry under stirring condition, continued for 20min (shown in tables 1,2). Adjust the pH of gel using phosphate buffer solution [13,14].
| Ethosomal formulation | Lecithin (Soya lecithin%) | Ethanol (%) | Propylene glycol (%) | Drug (g) | Cholesterol(g) | Water |
|---|---|---|---|---|---|---|
| EF1 | 2 | 20 | 10 | 0.080 | 0.005 | q.s |
| EF2 | 2 | 30 | 10 | 0.080 | 0.005 | q.s |
| EF3 | 2 | 40 | 10 | 0.080 | 0.005 | q.s |
| EF4 | 2 | 50 | 10 | 0.080 | 0.005 | q.s |
| EF5 | 3 | 20 | 10 | 0.080 | 0.005 | q.s |
| EF6 | 3 | 30 | 10 | 0.080 | 0.005 | q.s |
| EF7 | 3 | 40 | 10 | 0.080 | 0.005 | q.s |
| EF8 | 3 | 50 | 10 | 0.080 | 0.005 | q.s |
| EF9 | 4 | 20 | 10 | 0.080 | 0.005 | q.s |
| EF10 | 4 | 30 | 10 | 0.080 | 0.005 | q.s |
| EF11 | 4 | 40 | 10 | 0.080 | 0.005 | q.s |
| EF12 | 4 | 50 | 10 | 0.080 | 0.005 | q.s |
| *EF13 | - | - | - | 0.080 | 0.005 | q.s |
| *EF13 free drug suspension. | ||||||
Table 1: Composition of different ethosomal gel formulation.
| Gel formulation | Ciclopirox ethosomal suspension(ml) | Carbopol (%) | Triethanolamine (ml) | Phosphate buffer (pH 7.4) |
|---|---|---|---|---|
| EG-1 | 20 | 1.5 | 0.5 | QS |
| *EG-2 | 0.160g | 1.5 | 0.5 | QS |
| *EG-2 free drug gel. | ||||
Table 2: Composition of different ethosomal gel formulation.
Analytical study
Drug solubility: Cilcopirox drug solubility was studied in different solvents. It is observed that drug ciclopirox is sparingly soluble in water, soluble in ethanol, methanol and phosphate buffer 6.8pH [15].
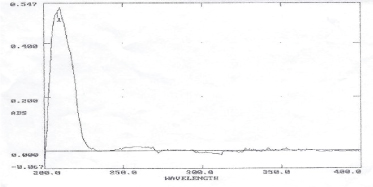
Drug lamda max determination: Ciclopirox drug scanned under UV visible spectrophotometer and it is observed that maximum absorbance at 212nm as shown in figure 2.
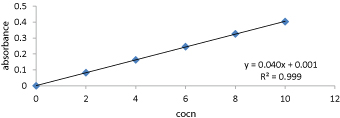
Preparation of calibration curve for Ciclopirox:Ciclopirox drug solutions were prepared in 2-10 μg/ml concentrations using 7.4pH phosphate buffer as solvent [16,17]. Absorbance noted for each concentration using UV Visible spectrophotometer at 212nm and plotted against concentration as shown in figure 3.
Drug – Excipients compatibility studies (FTIR):IR spectroscopy can be used to investigate and predict any physicochemical interactions between different components in a formulation and therefore it can be applied to the selection of suitable chemically compatible excipients [18- 20]. Drug – Excipients compatibility studies were shown in figures 4 and 5.
Evaluation Parameters
Evaluation of ethosomal vesicles
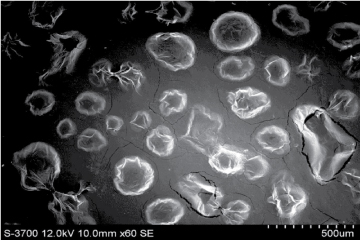
Vesicular characterization: Ethosomes were visualized by using Scanning electron microscopy (SEM). Effect of lecithin and ethanol concentrations on the size and distribution of ethosome vesicles was investigated using complete size distribution analysis.
Figure 2:UV Spectrum of Ciclopirox drug.
Figure 3:Calibration curve of Ciclopirox drug in pH7.4 phosphate buffer.
Figure 4:FTIR of Ciclopirox drug.
Figure 5:FTIR of optimized ethosomal gel.
| Formulation code | Entrapment efficiency (%) | Drug content (%) | PH | viscosity(cps) at 10 rpm | Spreadability (g.cm/sec)) |
|---|---|---|---|---|---|
| EF1 | 81.21 | 98.6 | 6.6 | 18124 | 16.84 |
| EF2 | 85.63 | 99.3 | 6.7 | 22776 | 15.92 |
| EF3 | 87.51 | 98.6 | 6.6 | 20460 | 17.01 |
| EF4 | 89.23 | 98.3 | 6.6 | 13359 | 15.10 |
| EF5 | 85.12 | 95.3 | 6.7 | 12107 | 19.38 |
| EF6 | 92.52 | 95.6 | 6.8 | 18723 | 18.41 |
| EF7 | 93.61 | 99.7 | 7.0 | 16590 | 20.30 |
| EF8 | 95.21 | 97.2 | 6.7 | 17421 | 17.62 |
| EF9 | 83.21 | 98.1 | 6.8 | 15421 | 17.21 |
| EF10 | 85.07 | 97.3 | 6.8 | 14321 | 18.18 |
| EF11 | 88.23 | 96.4 | 6.7 | 15234 | 18.26 |
| EF12 | 90.23 | 96.5 | 6.6 | 14231 | 17.25 |
| *Entrapment efficiency is evaluation test for ethosome suspension, remaining evaluation parameters for ethosomal gels. | |||||
Table 3: Evaluation test for ethosomes and ethosomal gels.
Figure 6:Scanning electron microscope image for ethosomes.
Drug entrapment efficiency: Ciclopirox entrapped within the vesicles was estimated by removing the unentrapped drug. Unentrapped drug was removed by centrifugation of dispersion at 22000rpm at a temperature of 4oC for 45minutes.
In-vitro evaluation of ethosomes: In-vitro drug release from ethosomes suspension was studied using Franz diffusion cell. The effective area for permeation was 1cm2 and diffusion cell receptor volume is 20ml. Egg membrane was used as semi permeable membrane between receptor and donor chambers in Franz diffusion cell. Phosphate buffer (7.4pH) was used as dissolution medium in receptor chamber. Temperature maintained at 370C and stirring of phosphate buffer in diffusion cell was maintained by using magnetic stirrer at 100rpm. 1-2ml samples were withdrawn through sample port of the Franz diffusion cell at predetermined time point upto 12-24hrs and replace same volume of fresh dissolution medium in diffusion cell.
Evaluation of ethosomal gel
pH of ethosomal gel: The pH of the gel was measured using a digital pH meter by dipping the glass electrode completely into the ethosomal gel formulation as to cover the electrodes.
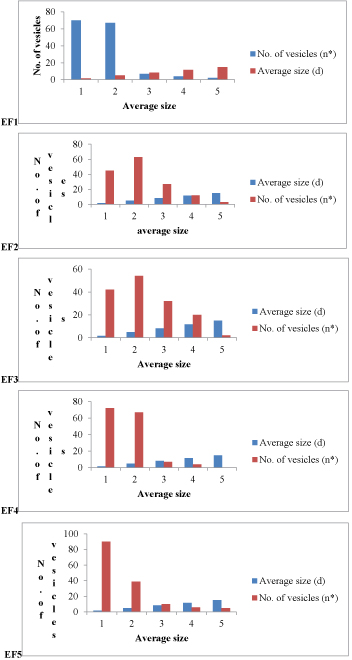
Figure 7:Size distribution analysis of formulations EF1-EF12. (d(avg) values for EF1=3.904 μm, EF2= 5.32 μm, EF3=5.79 μm, EF4=3.818 μm, EF5= 3.72 μm, EF6= 3.26 μm, EF7= 4.10 μm, EF8= 5.45 μm, EF9= 5.89 μm, EF10= 4.118 μm, EF11= 4.12 μm, EF12= 3.56 μm).
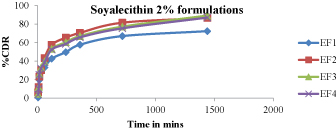
Figure 8:In -vitro drug release studies (EF1-EF4).
Viscosity of the ethosomal gel:Viscosity of ethosomal gel was measured using a Brookfield viscometer, average of 3 measurements was considered as the viscosity of the ethosomal gel.
Drug content:Weighed ethosomal gel was dissolved in methanol and filtered by filter paper to collect filtered drug solution. Filtered drug solution was analyzed using UV visible spectrophotometer at 212nm.
Spreadability:Spreadability was measured using an equipment in which one side was fixed on wooden block and upper side was movable it is tied with weight pan. To calculate spreadability 3-5grams of gel was placed between two slides, weights added to move the slide were noted in below formula.
Spreadability (g.cm/sec) = weight tide X length moved on glass side/ time taken to slide
Pharmacokinetic studies:In vitro dissolution data of optimized formulation was further investigated by using different release kinetic models.
Results and Discussion
Scanning electron microscope (SEM)
Figure 6
Size distribution analysis
Figure 7
Evaluation of ethosomes and ethosomal gels
Table 3
In-vitro drug release
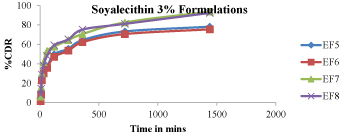
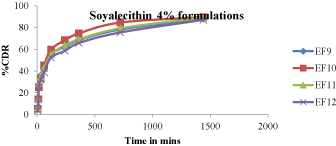
Figure 8 - 11
Figure 9:In -vitro drug release studies (EF5-EF8).
Figure 10:In -vitro drug release studies (EF9-EF12).
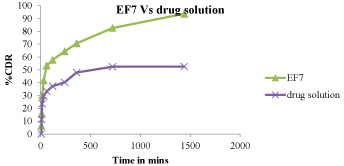
Figure 11:Drug release comparision between EF7 and EF13.
| ZERO | FIRST | HIGUCHI | PEPPAS | |
|---|---|---|---|---|
| % CDR Vs T | Log % Remain Vs T | %CDR Vs √T | Log C Vs Log T | |
| Slope | 2.657280722 | -0.05333783 | 14.23132743 | 0.69450863 |
| Intercept | 13.78182621 | 2.097605075 | 0.71982 | 0.921521334 |
| Correlation | 0.979600184 | -0.65822379 | 0.986315442 | 0.768192152 |
| R 2 | 0.959616521 | 0.433258566 | 0.932818151 | 0.590119183 |
Table 4:Release kinetics for optimized formulation.
Pharmacokinetic profile for EF7 ethosomal gel
Table 4
Conclusion
Ciclopirox ethosomes were designed by the method reported by Touitouetal., with few changes. Ethosomes were by specific software for sonicated ethosomes respectively. Vesicular size was found to be in the range of 0 – 5.89 μm. After verification of vesicles and their size, the drug is entraped by vesicular system and it was evaluated by ultra-centrifugation. Sonicated ethosomes containing 50% ethanol and Lecithin 3% showed higher entrapment value.
In-vitro release was carried out using egg membrane as semi permeable membrane. The optimized gel formulation was found to be zero order. % cumulative drug release into skin was also found to be maximum by the EF7 formulations.
Chien Yie W (1987) Transdermal Therapeutic systems, Conrolled Drug Delivery: Fundamentals & Applications. Marcel Dekker, New York.[ Ref ]
http://www.drugbank.com[ Ref ]
Divyesh P (2011) Transdermal drug delivery system: Review. International Journal of Biopharmaceutical and Toxicological Research 1: 61-80.[ Ref ]
Chien Yie W (1992) Novel drug Delivery Systems. Marcel Dekker, New York.[ Ref ]
Shailesh T, Prajapati, Charmi G, Patel, Chhagan N (2011) Formulation and Evaluation of Transdermal Patch of Repaglinide Transdermal patch of Repaglinide. ISRN pharm 2011: 651909.[ Ref ]
Jyotsana M, Argade, Kamal Dua (2015) Formulation and Evaluation of Transdermal Patches of Donepezil. Recent Patents on Drug Delivery & Formulation 9: 95-103.[ Ref ]
Ashish DM, Patel CN, Dinesh RS (2013) Formulation and Optimization of Ethosomes for Transdermal Delivery of Ropinirole Hydrochloride. Current Drug Delivery 10: 500-516.[ Ref ]
Bala Tripura Sundari, Sailaja Rao, Sireesha, Krishna Sai (2017) Formulation and Evaluation of ethosomal gels of mangifera indica leaf extract. IAJPS 4: 1755-1761.[ Ref ]
James KC (1986) Solubility and related phenomena. Marcel Dekker Inc, New York.[ Ref ]
Shingade G (2012) Review on: Recent trend on transdermal drug delivery system. J Drug Deliv Ther 2: 66-75.[ Ref ]
Anish, Raghavendra Dubey, Prabhat Jain (2019) Formulation and evaluation of Ethosomal gel of Tazarotene for topical delivery. Asian Journal of Pharmaceutics 13: 37-45.[ Ref ]
Sheo Datta M, Sunil kumar (2010) Formulation Development and evaluation of ethosome of Stavudine. Indian J Pharm Edu Res 44: 102- 108.[ Ref ]
Palliyil B, Lebo DB, Patel PR (2013) A preformulation strategy for the selection of penetration enhancers for a transungual formulation. AAPS PharmSciTech 14: 682-691.[ Ref ]
Tunyaluk L, Prapaporn B (2017) Ethosomes of phenylethyl resorcinol as vesicular delivery system for skin lightening applications. Biomed Research International.[ Ref ]
David SRN, Hui MS (2013) Formulation and in vitro evaluation of ethosomes as vesicular carrier for enhanced topical delivery of isotretinoin. International journal of drug delivery 5: 28-34.[ Ref ]
Williams AC, Barry BW (2004) Penetration enhancers. Advanced drug delivery review 56: 603-618.[ Ref ]
Bendas ER, Tadros MI (2007) Enhanced transdermal delivery of salbutamol sulfate via ethosomes. AAPS pharmaceutical sciences and technology 8: E1-E9.[ Ref ]
Sagar SB (2013) Design and development of ethosomal transdermal drug delivery system of valsartan with preclinical assessment in Wistar albino rats. Journal of Liposome research 23: 119-125.[ Ref ]
Roohi K, Dilip P, Aupam KS (2015) Ethosomes: A novel approach for transdermal and topical drug delivery. Research journal of topical and cosmetic science.[ Ref ]
Poonam V, Pathak K (2010) Therapeutic and cosmeceutical potential of ethosomes; An overview. J Adv pharm Technol Res 1: 274-282.[ Ref ]