Journal Name: Journal of Drug Development and Delivery
Article Type: Research
Received date: 03 July, 2018
Accepted date: 24 August, 2018
Published date: 28 August, 2018
Citation: Kinthada PMMS, Gust R(2018) Synthesis and Characterization, Nuclease Activity Studies and Anti-Cancer Studies of Au(III)-IsatinThiosemicarbazone Complexes as Potential Anticancer Drugs. J Drug Dev Del. Vol: 1, Issu: 1 (01-06).
Copyright: © 2018 Kinthada PMMS. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Cancer is an extremely deadly disease. It is undoubtedly one of the main health concerns facing our society and one of the primary targets regarding medicinal chemistry. Even though Platinum based complexes had been in primary focus on research on chemotherapy agents, the interests in this field have shifted to non-platinum based agents in order to find different metal complexes with less side effects and similar or better cytotoxicity. Thus a variety of metal complexes based on GOLD are being intensive studied as platinum replacements. There is need to develop these gold complexes as they are isoelectronic with platinum and these could someday become the replacement for Platinum based anticancer Drugs.
Keywords
Isatin, ISATIN.TSC, Chemoprevention, Therapy, Human malignancy.
Abstract
Cancer is an extremely deadly disease. It is undoubtedly one of the main health concerns facing our society and one of the primary targets regarding medicinal chemistry. Even though Platinum based complexes had been in primary focus on research on chemotherapy agents, the interests in this field have shifted to non-platinum based agents in order to find different metal complexes with less side effects and similar or better cytotoxicity. Thus a variety of metal complexes based on GOLD are being intensive studied as platinum replacements. There is need to develop these gold complexes as they are isoelectronic with platinum and these could someday become the replacement for Platinum based anticancer Drugs.
Keywords
Isatin, ISATIN.TSC, Chemoprevention, Therapy, Human malignancy.
Introduction
Inorganic chemotherapy has been considerably boosted in recent years by the discovery and exploitation of Anti-tumor compounds based on platinum. Some Gold (III) complexes display similar chemistry and pharmacological activity to cis-platin and its analogues are now the basis of multimillion dollars’ industry and bring benefit to thousands of cancer patients. Platinum based anticancer drugs induces normal tissue toxicity particularly to the kidneys and thus alternative metal compounds are presently being evaluated in clinical trials. Gold is one of the most promising metals and its compounds are regarded as promising alternatives in cancer therapy and offer many approaches to innovative Metallo-pharmaceuticals.
The Isatin molecule (1H-indole-2, 3-Dione) is a versatile moiety that displays diverse biological activities, including anticancer activity. N-alkylated indoles have also been reported to exhibit anticancer activity. For example, the indolyl amide D-24851 has been found to be block cell cycle progression in a variety of malignant cell line including those derived from the prostate, brain, breast, pancreas and colon. Indoles derivative vincristine and vinblastine is mainly useful for treating Hodgkin’s disease, lymphocytic lymphoma, histiocytic lymphoma, advanced testicular cancer, advanced breast cancer. The derivatives of Isatin- 3-thiosemicarbazones exhibit a broad spectrum of biological activity. They are active against Variola, Influenza viruses and some of these derivatives exhibit anticancer, antihistamine and antibacterial activity. Various classes of metal complexes of Isatin -3-thiosemicarbazone derivatives with Au, Ru, Sn, Pd, Ti, and Cu have been investigated during the last three decades as potential anticancer agents.
In this work we describe the synthesis and characterization of Gold (III)-IsatinThiosemicrbazone Complexes. A full range of gold compounds are currently being investigated for their potential as anti-tumor agents toward several human tumor cell lines and would be evaluated in vitro using a systematic screening strategy for their utilization in cancer therapy. The structural features from the spectroscopic and other studies qualify Gold (III) compounds as a promising class of cytotoxic agents of outstanding interest or cancer treatment.
Materials and Methods
All the reagents used in the preparation of ligands and their metal complexes were of reagent grade (Merck). The solvents used for the synthesis of ligands and metal, complexes were distilled before use. All other chemicals were of AR grade and used without further purification. The elemental analyses were performed by using micro analytical techniques. Gold (III) was estimated by using AAS model Z-6100 (Hitachi Ltd.,). Chlorine is estimated by using standard procedures. The IR spectra were recorded in the range 4000-200cm-1 using KBr discs with PerkinElmer model 1430 and 337. The electrical conductivity measurements were made in DMF (10–3 M) at room temperature (27 ± 20 c) using a Digisun digital conductivity meter (DI-909 model). The NMR spectra were recorded in DMSO-d6 on NMR spectrophotometer model JEOL Ex-90 FT using TMS as the reference. Mass spectrometer operating under liquid secondary ion mass spectral (LSIMS) conditions. The magnetic susceptibilities were determined at Room temperature, on a Guoy balance using Mercury Tetrathiocyanato Cobalt (II) as a magnetic standard. Molecular weights of the complexes were determined by cryoseopic method using camphor as solvent. Magnetic measurements were carried, out in the polycrystalline state on a PAR model ISS vibrating sample magnetometer operating at field strength of 2-1.0 KG.
Synthesis of ligands
Isatin thiosemicarbazones and substituted Isatin Thiosemicarbazones were synthesized by condensing equimolar solutions (0.01M) of Isatin and thiosemicarbazide / 4-substituted thiosemicarbazides in methanol in the presence of few drops of acetic acid. The solution after mixing was refluxed on water bath for 2hrs, filtered and the filtrate was concentrated to half the volume. Crystals were obtained on cooling the solution. The crystals were separated and recrystallized from Ethanol.
Synthesis of metal complexes
A Solution of AuCl3 was added to ligands (10-3 mole) in ethanol and the reaction mixture was refluxed for 3 hrs. The resulting colored precipitate was washed several times with methanol and the resulting precipitate was dried in a desiccator over anhydrous CaCl2.
Results and Discussion
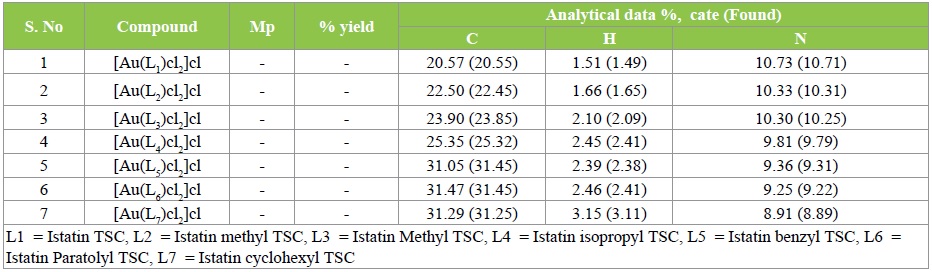
The analytical data of the synthesized Au (III)-Isatin Thiosemicarbazone complexes is given in Table 1.
IR spectra
The IR Spectral data of the synthesized Au (III)-Isatin Thiosemicarbazone complexes is given in Table 2.
In case of all complexes, the spectra reveal that the bond having a maximum at 1740 cm-1 in the free ligand is shifted to lower wave number, the shift being 26-35 cm-1. This shift indicates that the carbonyl oxygen atom of the Isatin residue is one of the coordinating sites. The band due to vNH vibration mode in Isatin having maximum at 3190 cm-1 in the free ligand, remains largely unaffected in the chalates indicating thereby the non-participation of this group in coordination. This shifting of v (c=s) 874 cm1 towards lower side by v 20-30 cm-1 suggests the involvement of Sulphur in coordination. We can therefore conclude that the ligands act as bidentate, the coordination occurring through v (C = S) and v (C=0).
HNMR spectra
Supplementary data have been obtained by 1H and 13C NMR Spectra which were recorded for the ligands and their Au (III) complexes. HNMR Spectra of the ligand can be resolved into three distinct regions. The spectra exihibit two multiplets at 6.94-7.68 and 6.3 – 6.8 pm due to the Isatin and amine aromatic rings respectively and one singlet at 10.3 ppm due to the Isatin NH residue. The only HNMR signal displaying a downfield shift in complex compounds (from 10.3 to 11.24 pm) are those associated with the hydrogen of the Isatin NH residue. This behavior is related with a decrease of the election density and DE shielding of the NH Proton as a result of the participation of the adjacent carbonyl oxygen in coordination [5,6]. This behavior is in good agreement of the >C = 0 vibration mode appear at regular with the corresponding free ligands.
Electron spin resonance spectra
Electronic spin Resonance spectra of Gold complex was recorded in DMF at liquid nitrogen temperature value equal to 22. Electronic resonance spectra of gold complexes were measured in DMF and liquid nitrogen temperature.
Electronic spectra
Since All the Gold(III) complexes are Diamagnetic, they did no0t show any bands in electronic spectrum.
Magnetic studies
All the complexes are diamagnetic. Molar Conductance studies: The molar conductivity data suggest that the complexes are 1:1 electrolyte. The magnetic moments (Table 1) of metal complexes are found to be subnormal which may be attributed to the presence of magnetically coupled metal centers in dimeric complexes.
Table 1: Analytical data of isatin thiosemicarbazone complexes.
Nuclease activity studies
The nuclease activity of present ligands and their - complexes has been investigated on pBR 322 plasmid DNA by agarose gel electrophoresis in the presence / absence of H2O2. At micro molar, concentration, the ligands exhibit no significant activity in absence and in the presence of the oxidant as shown in Figure 1. The nuclease activity is greatly enhanced by incorporation of metal ions m the ligands. In absence of oxidants, the Gold(III)-Complexes of Isatin and Substituted Isatin thiosemicarbazone’s causes discernible DNA cleavage as evidenced by increase in intensity in form 11 (nicked) and form III (linear) with decrease in intensity in from 1 (super coiled) which is attributed to step-wise conversion of from I to form II and to form III Similar observations were also evident in the Gold (III)- Complexes of Isatin Thiosemicarbazone and the conversion to linear form was complete. The nuclease activity of the Gold (III) complexes with Isatin Thiosemicarbazones is more when compared to other Metal Complexes of Isatin Thiosemicarbazones.
Figure 1: Au (III) TSC complex.
The Nuclease activity of present Ligands and their complexes has been investigated on pBR 322 plasmid DNA by Agarose Gel Electrophoresis in the presence/Absence and in the presence of Oxidant.The Nuclease activity is greately enhanced by incorporation of Metal Ion in the ligands. In the absence of Oxidants Au(III) complexes of BAMOT and Substituted Isatin Thiosemicarbazones cause Discernible DNA cleavage as was evidenced increase in form-II(Nicked) and form-III(linier) with decrease in intensity in Form-I (super coiled) which is attributed to stepwise conversion of Form-I to form-II and to form-III. All complexes show much enhanced nuclease activity in the presence of oxidant, which may be due to free radical reaction (OH*) with DNA. The production of hydroxyl radicals due to the reaction between H202 and the metal complexes. The OH* radical involves oxidation of deoxyribose moiety followed by hydrolytic cleavage of sugar phosphate backbone. The higher activity of Isatin Thiosemicarbazone complexes is probably due to presence of lipophilic –CH3 group. The lipophilic nature is evaluated, by thin layer L chromatography for the ligands in 10- 3 N N M HN S H2N O CH3 H3C S N NH NH2 H3C CH3 O N M n 2Y 2 - + x x x x M alcoholic solutions. The stationary phase was silica gel chemically bonded (Nano-Si NH2) and mobile phase was a 2:3 mixtures of water and methanol. The Rf values [ISATIN THIOSEMICARBAZONES – around 46 in mm] indicate more lipophilic nature of Isatin Thiosemicarbazone and its complexes.
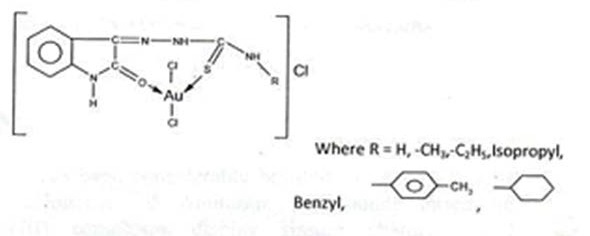
From above discussion it is proposed that all Au (III) TSC complexes are square planner in geometry, and the structure of the complexes have been proposed as shown in the figure below:
These Au(III)-Isatin Thiosemicarbazone complexes have been screened for their Anti-cancer activity and other pertinent studies have been done and reported.
Anticancer activity studies
Antiproliferative effects: In vitro cytotoxicity assays were performed to get an insight into the antitumor activity of the novel gold complexes. All complexes as well as the established antitumor drug cisplatin were screened against MCF-7 and MDA-MB 231 breast cancer cells according to established procedures [7-9]. IC50 values determined after 72 h incubation are shown in Table 2.
Table 2: Cisplatin and the cell growth.
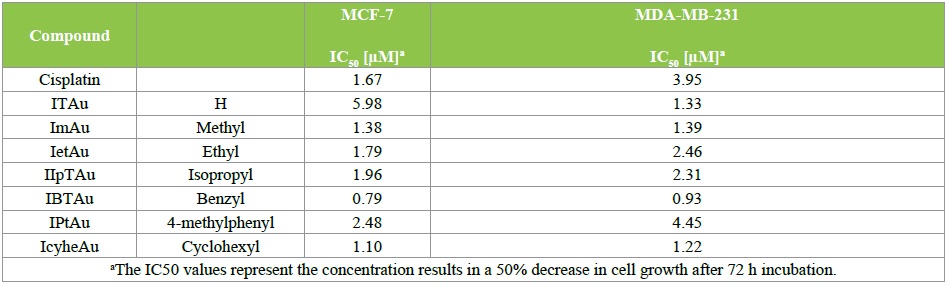
Cisplatin characteristically reduced the cell growth of MCF-7 (IC50 = 1.67 μM) and MDA-MB 231 (IC50 = 3.95 μM) cells.
The lead compound ITAu was distinctly less active than cisplatin at the MCF-7 cell line (IC50 = 5.98 μM), while it was 3 fold more active against MDA-MB-231 cells (IC50 = 1.33 μM). The cytotoxicity of the isatin gold complexes depended on the substituent at the thiosemicarbazone moiety. An N-methyl group increased the activity against MCF-7 cells (IC50 = 1.38 μM) but did not change activity at the MDAMB- 231 cell line. Interestingly, N-ethyl or N-isopropyl chains reduced the growth inhibitory effects only at MDA-MB-231 cells (IC50 = 2.46 and 2.31 μM, respectively). However, both compounds are still more effective than cisplatin. The best results were achieved with the benzyl derivative. IBTAu caused growth inhibition of MCF-7 and MDA-MB-213 cells at nanomolar concentrations (IC50 = 0.79 and 0.93 μM). Exchange of the benzyl residue by p-toluolyl moiety (IPTAu) strongly reduced the cytotoxic potency to IC50 = 2.48 and 4.45 μM, respectively. It is to metion that IcyheAu with an N-cyclohexyl moiety (IC50 = 1.10 and 1.22 μM) was only marginally less active than IBTAu. These data clearly document that the in vitro antitumor potency and the terminal N-substituents determine the tumor selectivity.
Cellular and nuclear uptake as well as DNA interaction: Because the cytotoxic effects of metal complexes in cell culture experiments were strongly influenced by cellular uptake and accumulation, the intracellular drug level in MCF-7 and HT-29 cells was studied on the example of ITAu and IBTAu.
In the present study we used a recently described electrothermal atomic absorption spectroscopy (ETAAS) method, which was developed for the bioanalysis of gold complexes and based on the standard addition method [7]. Experiments were performed using an extracellular concentration of 10 μM and a short exposure period of 4 h to avoid a loss of cell biomass due to toxic effects. Results were corrected for the respective protein contents of the samples and values are accordingly presented as ng of metal/mg of protein.
As listed in Table 3, ITAu and IBTAu differ in the cellular uptake. The gold content in MCF-7 cells incubated with IBTAu was 3 fold higher compared to those incubated with ITAu (IBTAu, 132.5 ng/mg; ITAu, 36.00 ng/mg). Interestingly, IBTAu was 2-fold higher accumulated in MDA-MB-231 (264.9 ng/mg) than in MCF-7 cells (132.5 ng/mg), while the gold content reached with ITAu was nearly the same in both cell lines (36.00 and 42.72 ng/mg, respectively).
Table 3: ITAu and IBTAu in the cellular uptake.
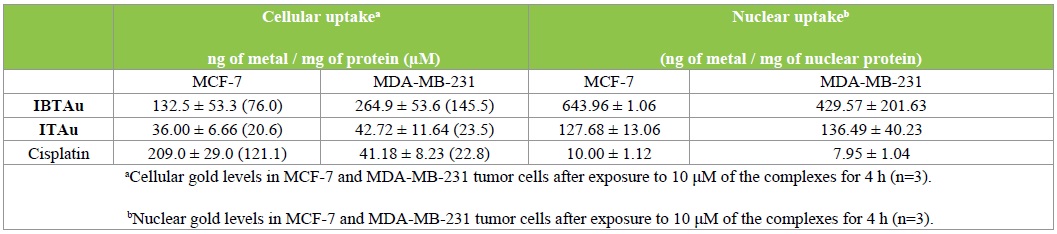
This means that IBTAu showed an accumulation grade of 7.6 in MCF-7 and 14.6 in MDA-MB-231 cells if the individual cellular parameters were taken into account [4]. Under the same conditions ITAu was only 2.1 and 2.3 fold accumulated, respectively. It is worthy to mention that the intracellular metal concentrations in MCF-7 cells achieved with both compounds were low compared to cisplatin (209 ng/mg) while the content of AITAu in MDA-MB 231 cells was identical to cisplatin (41.18 ng/mg) and that of IBTAu was even 6.5 time higher.
To get information if the complexes might reach the major target of metal complexes, the DNA, the nuclei of MCF-7 and MDA-MB 231 cells treated with IBTAu and ITAu or cisplatin were isolated by a short sucrose gradient and investigated for their gold content using ETAAS. The results were calculated as ng of Au/mg of nuclear protein (Table 2).
Cisplatin caused only a low metal content in the nuclei nearly independent on the used cells (MCF-7: 10.00 ng/mg, MDA-MB 231: 7.95 ng /mg). In the nuclei of cells treated with IBTAu a 50-60 fold higher metal concentration was detected (MCF-7: 643.96 ng/mg, MDA-MB 231: 429.57 ng /mg). Cells treated with ITAu showed a gold level of 127.68 ng/mg (MCF-7) and 136.49 ng/mg (MDA-MB 231), respectively. This finding points to DNA targeting with gold complexes bearing an isatin ligand. Furthermore, it is very likely that the substituents at the terminal amino group determine cellular and nuclear uptake. If the DNA binding related to the mode of action of the gold complexes is still unclear, because the nuclear metal contents do not correlate with the growth inhibitory effects. The marked “soft” character of the gold (I) center makes on the one hand a selective and tight reaction with the nitrogen donors of nucleobases rather unlikely. On the other hand, recent studies revealing strong interactions with specific protein side chains such as thiols and selenols indirectly support this assumption [11,12]. However, to make a final statement on the mode of action further investigations are necessary.
Experimental part
Cytotoxicity: The human MCF-7, MDA-MB 231 breast and HT-29 colon cancer cell lines were obtained from the American Type Culture Collection. All cell lines were maintained as a monolayer culture in L-glutamine containing Dulbecco’s modified Eagle’s medium (DMEM) with 4.5 g/L glucose (PAA Laboratories, Austria), supplemented with 5% fetal bovine serum (FBS; Biochrom, Germany) in a humidified atmosphere (5% CO2) at 37°C.
The experiments were performed according to established procedures with some modifications.1,2,3 In 96 well plates 100 μL of a cell suspension in culture medium at 7500 cells/mL (MCF-7 and MDA-MB 231) or 3000 cells/mL (HT-29) were plated into each well and incubated for three days under culture conditions. After the addition of various concentrations of the test compounds, cells were incubated for up to appropriate incubation time. Then the medium was removed, the cells were fixed with glutardialdheyde solution 1% and stored under phosphate buffered saline (PBS) at 4°C. Cell biomass was determined by a crystal violet staining, followed by extracting of the bound dye with ethanol and a photometric measurement at 590 nm. Mean values were calculated and the effects of the compounds were expressed as % Treated/Controlcorr values according to the following equations:
(C0 control cells at the time of compound addition; C control cells at the time of test end; T probes/samples at the time of test end).
The IC50 value was determined as the concentration causing 50% inhibition of cell proliferation and calculated as mean of at least two or three independent experiments (OriginPro 8).
Sample Preparation for Cellular Uptake Studies. The cellular uptake was measured according to a previously described procedure.1 In short: cells were grown until at least 70% confluency in 175 cm2 cell culture flasks. Stock solutions of the gold complexes in DMF were freshly prepared and diluted with cell culture medium to the desired concentration (final DMF concentration: 0.1% V/V, final gold complex concentration: 10.0 μM). The cell culture medium of the cell culture flasks was replaced with 10 mL of the cell culture medium solutions containing the compounds and the flasks were incubated at 37 °C/5% CO2 for 6 h. The cell pellets were isolated by trypsinisation and centrifugation (room temperature, 2000 rpm, 5 min), resuspended in twice distilled water, lysed by using a sonotrode and appropriately diluted using twice distilled water. An aliquot was removed for the purpose of protein quantification by the Bradford method. The determination of the gold content of the samples was performed by ETAAS. Results were calculated from the data of three independent experiments and are given as ng gold per mg cellular protein.
Sample preparation for nuclear uptake studies: The nuclei of the tumor cells were isolated according to previously described procedures [1] with some minor modifications: cells were grown in 175 cm2 cell culture flasks until at least 70% confluency. The medium was removed and replaced with 10 mL of medium containing 10.0 μM drug. After 24 h of incubation at 37°C in humidified atmosphere, the drug containing medium was removed, cells were trypsinized, resuspended in 10 mL of cell culture medium, isolated by centrifugation (1500 rpm, 5 min) and 0.5–1.0 mL of 0.9% NaCl solution were added. After centrifugation (1500 rpm, 5 min) pellets were resuspended in 300 μL of RSB-1 (0.01 M Tris–HCl, 0.01 M NaCl, 1.5 mM MgCl2, pH 7.4) and left for 10 min in an ice-bath. Swollen cells were centrifuged (2000 rpm, 5 min), resuspended in 300 μL of RSB-2 (RSB-1 containing each 0.3% V/V Nonidet-P40 and sodium desoxycholate) and homogenized by 10–15 up/ down-pushes in a 1 mL syringe with needle. Aliquots of 50 μL of the homogenisate were removed for determination of the total gold content and mixed with 500 μL water. The homogenisate was centrifuged at 2500 rpm for 5 min and the resulting crude nuclei were taken up in 150 μL of 0.25 M sucrose containing 3 mM CaCl2. The suspension was underlayed with 150 μL of 0.88 M sucrose and centrifuged 10 min at 2500 rpm. The nuclei pellets were stored at –20°C or immediately dissolved in 500 μL water and disrupted by use of a sonotrode. The gold content of the samples was measured by ETAAS and the protein content by the Bradford method. Results are expressed as means of three independent experiments as ng gold per mg nuclear protein.
Atomic absorption spectroscopy (AAS): ETAAS measurements were performed according to a previously published standard addition procedure with some minor modifications [1]. In short: to 100 μL aliquots of the diluted lysates increasing amounts of aqueous gold standard solutions were added. All probes were adjusted to a final volume of 200 μL using twice distilled water, each 20 μL triton X-100 (1%) and ascorbic acid (1%) were added and the probes were measured as described below. The gold content of the lysates was accessed by the linear extrapolation method. A Vario [6] electro thermal atomic absorption spectrometer (AnalytikJena AG) was used for the gold measurements. Gold was detected at a wavelength of 242.8 nm with a band pass of 0.8 nm. A deuterium lamp was used for background correction. Probes were injected at a volume of 25 μL into regular graphite wall tubes. Drying, atomization and tube cleaning steps were performed as outlined in more detail in the literature.1 The temperature for pyrolysis was set to 1200°C. The mean AUC (area under curve) absorptions of duplicate injections were used throughout the study. The limit of gold detection using biological samples as described above was 1.7 μg/L.
Acknowledgements
The author, KMMSP thanks the National Institute of Health (NIH) authorities, Maryland, USA for awarding a NIH Visiting Fellowship for Post-Doctoral research at Karmanos Cancer Research center, Wayne State University, School of Medicine, Detroit, Michigan, USA.
There is no references