Journal Name: Journal of Drug Development and Delivery
Article Type: Research
Received date: 09 January, 2019
Accepted date: 18 January, 2019
Published date: 25 January, 2019
Citation: Ahmed SH, Karunakranth D, Babu RS, Khasim SM, Khan MA, et al (2019) To Develop New RP HPLC Method for the Simultaneous Estimation of Tamsulosin Hydrochloride and Dutasteride in Pharmaceutical Dosage Form. J Drug Dev Del Vol: 2, Issu: 1 (07-12).
Copyright: © 2019 Ahmed SH, et al. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
A simple and selective LC method is described for the determination of Tamsulosin Hydrochloride and Dutasteride in pharmaceutical dosage form. Chromatographic separation was achieved on a c18 column using mobile phase consisting of a mixture of 40 volumes of 20mM Ammonium acetate buffer pH 3.5:30 volumes of Acetonitrile: 30 volumes of Methanol with detection of 223nm. Linearity was observed in the range 19.2-44.8 µg/ml for Tamsulosin Hydrochloride (r² = 0.996) and 24-56 µg/ml for Dutasteride (r² = 0.998) for the amount of drugs estimated by the proposed methods was in good agreement with the label claim.
The proposed methods were validated. The accuracy of the methods was assessed by recovery studies at three different levels. Recovery experiments indicated the absence of interference from commonly encountered pharmaceutical additives. The method was found to be precise as indicated by the repeatability analysis, showing % RSD less than 2. All statistical data proves validity of the methods and can be used for routine analysis of pharmaceutical dosage form.
Keywords:RP HPLC, Tamsulosin Hydrochloride, Dutasteride, Mobile phase
Abstract
A simple and selective LC method is described for the determination of Tamsulosin Hydrochloride and Dutasteride in pharmaceutical dosage form. Chromatographic separation was achieved on a c18 column using mobile phase consisting of a mixture of 40 volumes of 20mM Ammonium acetate buffer pH 3.5:30 volumes of Acetonitrile: 30 volumes of Methanol with detection of 223nm. Linearity was observed in the range 19.2-44.8 µg/ml for Tamsulosin Hydrochloride (r² = 0.996) and 24-56 µg/ml for Dutasteride (r² = 0.998) for the amount of drugs estimated by the proposed methods was in good agreement with the label claim.
The proposed methods were validated. The accuracy of the methods was assessed by recovery studies at three different levels. Recovery experiments indicated the absence of interference from commonly encountered pharmaceutical additives. The method was found to be precise as indicated by the repeatability analysis, showing % RSD less than 2. All statistical data proves validity of the methods and can be used for routine analysis of pharmaceutical dosage form.
Keywords:RP HPLC, Tamsulosin Hydrochloride, Dutasteride, Mobile phase
Introduction
Tamsulosin
Tamsulosin is a selective antagonist at alpha-1A and alpha-1Badrenoceptors in the prostate, prostatic capsule, prostatic urethra, and bladder neck. At least three discrete alpha1-adrenoceptor subtypes have been identified: alpha-1A, alpha-1B and alpha-1D; their distribution differs between human organs and tissue [1]. Approximately 70% of the alpha1-receptors in human prostate are of the alpha-1A subtype. Blockage of these receptors causes relaxation of smooth muscles in the bladder neck and prostate.
Dutasteride
Dutasteride belongs to a class of drugs called 5-alphareductase inhibitors, which block the action of the 5-alpha-reductase enzymes that convert testosterone into dihydrotestosterone (DHT). Finasteride also belongs to this group, but while Dutasteride inhibits both isoforms of 5-alpha reductase, finasteride inhibits only one. Even so, a clinical study done by GlaxoSmithKline, the EPICS trial, did not find Dutasteride to be more effective than finasteride in treating BPH [5].
Mechanism of action: Dutasteride inhibits the conversion of testosterone to 5 alpha-dihydrotestosterone (DHT), which is the androgen primarily responsible for the initial development and subsequent enlargement of the prostate gland. Testosterone is converted to DHT by the enzyme 5 alpha-reductase, which exists as 2 isoforms, type 1 and type 2 [6]. Dutasteride is a competitive and specific inhibitor of both type 1 and type 2, 5-alpha-reductase isoenzymes, with which it forms a stable enzyme complex. Dissociation from this complex has been evaluated under in vitro and in vivo conditions and is extremely slow. Dutasteride does not bind to the human androgen receptor [7,8].
Aim and Objective
Aim
To develop new RP HPLC method for the simultaneous estimation of Tamsulosin Hydrochloride and Dutasteride in pharmaceutical dosage form.
Objective
• Solubility determination of Tamsulosin Hydrochloride and Dutasteride in various solvents and buffers.
• Determine the absorption maxima of both the drugs in UV– Visible region in different solvents/buffers and selecting the solvents for HPLC method development.
• Optimize the mobile phase and flow rates for proper resolution and retention times.
• Validate the developed method as per ICH guidelines.
Materials and Methods
Tables 1 and 2
Mobile phase
A mixture of 40 volumes of 20mM Ammonium acetate buffer pH 3.5:30 volumes of Acetonitrile: 30 volumes of Methanol. The mobile phase was sonicated for 10 min to remove gases.
Determination of working wavelength (λ Max)
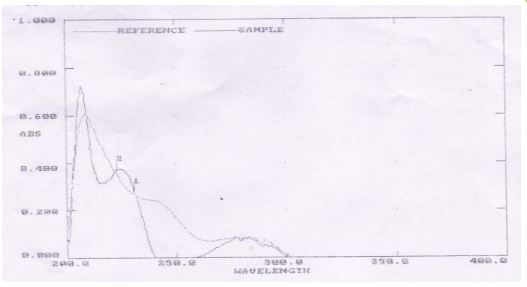
In simultaneous estimation of two drugs isobestic wavelength is used. Isobestic point is the wavelength where the molar absorptivity is the same for two substances that are interconvertible. So this wavelength is used in simultaneous estimation to estimate both drugs accurately.
Preparation of standard stock solution of Tamsulosin Hydrochloride
25 mg of Tamsulosin Hydrochloride was weighed and transferred in to 250ml volumetric flask and dissolved in methanol and then make up to the mark with methanol and prepare 10 µg/ml of solution by diluting 1ml to 10ml with methanol.
Preparation of standard stock solution of Dutasteride
25mg of Dutasteride was weighed in to 250ml volumetric flask and dissolved in Methanol and then dilute up to the mark with methanol and prepare 10 µg/ml of solution by diluting 1ml to 10ml with methanol.
Were soluble it was used as solvent for λ max determination by UV-Visible Spectroscopy.
Assay
Preparation of samples for assay
Preparation of mixed standard solution: Standard stock solutions of Tamsulosin Hydrochlorideand Dutasteride (microgram/ml) were prepared by dissolving 40 mg of Tamsulosin Hydrochloride and 32 mg of Dutasteride dissolved in sufficient mobile phase. After that filtered the solution using 0.45-micron syringe filter and Sonicated for 5 min and dilute to 100 ml with mobile phase. Further dilutions are prepared in 5 replicates of 40 μg/ml of Tamsulosin Hydrochlorideand 32 μg/ml of Dutasteride was made by adding 1 ml of stock solution to 10 ml of mobile phase.
Sample preparation: 10 tablets (each tablet contains 0.5 mg of Tamsulosin Hydrochloride and 0.4 mg of Dutasteride) were weighed and taken into a mortar uniformly mixed. Test stock solutions of Tamsulosin Hydrochloride (40 μg/ml) and Dutasteride (32 μg/ml) were prepared by dissolving weight equivalent to 40 mg of Tamsulosin Hydrochloride and 32 mg of Dutasteride and dissolved in sufficient mobile phase. After that filtered the solution using 0.45-micron syringe filter and Sonicated for 5 min and dilute to 100ml with mobile phase. Further dilutions are prepared in 5 replicates of 40 μg/ml of Tamsulosin Hydrochloride and 32 μg/ml of Dutasteride was made by adding 1 ml of stock solution to 10 ml of mobile phase.
| Water | HPLC Grade |
|---|---|
| Methanol | HPLC Grade |
| Potassium Dihydrogen Phosphate | AR Grade |
| Acetonitrile | HPLC Grade |
| Dipotassium hydrogen phosphate | AR Grade |
| Acetonitrile | HPLC Grade |
Table 1: Reagents used
| QUINAPRIL AND TOLCAPONE drugs | Gift Samples obtained from Chandra labs, Hyd. |
|---|---|
| PFIZA (QUINAPRIL- 10mg & TOLCAPONE- 12.5) Tablet dosage form | Obtained from local pharmacy |
Table 2: Drugs used.
| QUINAPRIL | TOLCAPONE | |||
|---|---|---|---|---|
| Injection-1 | 1136.114 | 1120.050 | 2576.974 | 2541.448 |
| Injection-2 | 1112.446 | 1121.051 | 2535.582 | 2551.500 |
| Injection-3 | 1115.176 | 1123.043 | 2549.337 | 2545.160 |
| Injection-4 | 1116.202 | 1118.821 | 2538.795 | 2551.600 |
| Injection-5 | 1124.282 | 1112.446 | 2544.742 | 2535.582 |
| Average Area | 1120.844 | 1118.942 | 2549.086 | 2545.058 |
| Standard deviation | 3.615683 | 6.83985 | ||
| %RSD | 0.323134 | 0.26875 | ||
| Assay(%purity) | 99.83032 | 99.84198 | ||
Table 3: Assay results.
Calculation
The amount of TOLCAPONE and QUINAPRILpresent in the formulation by using the formula given below, and results shown in table 3.
Where,
AT = Peak area of sample preparation,
AS = Average Peak area of standard preparation,
WS = Weight of drug in mg,
DS & DT = Dilution of standard and sample preparation,
WT = Weight of Sample in Assay preparation,
P = Percentage purity of working standard,
LC = Label Claim of drug.
Observation: The amount of Tamsulosine Hydrochloride and Dutasteride present in the taken dosage form was found to be 98.93 % and 99.16% respectively.
Method validation
Validation: Validation is a process of establishing documented evidence, which provides a high degree of assurance that a specific activity will consistently produce a desired result or product meeting its predetermined specifications and quality characteristics. Method validation is the process of demonstrating that analytical procedures are suitable for their intended use and that they support the identity, quality, purity and potency of the drug substances and drug products.
Validation parameters
a) Specificity / Selectivity
b) Accuracy
c) Precision
d) Linearity & Range
e) Limit of Detection
f) Limit of Quantitation
g) Robustness
h) Ruggedness
i) System Suitability
Results and Discussion
Wavelength optimization by UV– spectroscopy
Figure 1
Method development and optimization of RP-HPLC method
Table 4
Method validation
System suitability: Standard solutions were prepared as per the test method and injected into the chromatographic system. The system suitability parameters like theoretical plates, resolution and asymmetric factor were evaluated.
Tables 5 and 6
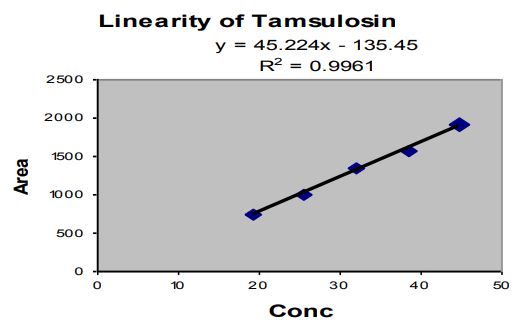
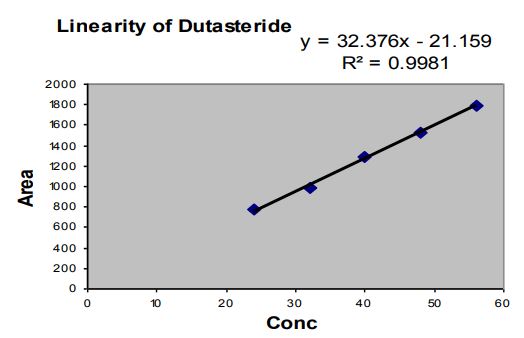
Linearity
Tables 7,8 and 9
Figures 2 and 3
Observation: The correlation coefficient for linear curve obtained between concentration vs. Area for standard preparations of Tamsulosin Hydrochloride and Dutasteride is 0.9961 and 0.9981.
| Mobile phase | Methanol:Water |
|---|---|
| Ph | - |
| Column | Inertsil ODS 3V column,C18(150x4.6 ID) 5µm |
| Flow rate | 1.0 ml/min |
| Column temperature | Room temperature(20-25°C) |
| Sample temperature | Room temperature(20-25°C) |
| Wavelength | 220 |
| Injection volume | 20 µl |
| Run time | 6 min |
| Retention time | About 2.707 min for Quinapril and 3.953 min for Tolcapone. |
Table 4: Optimized chromatographic conditions
Figure 1: Wavelength optimization by UV– spectroscopy
| Injection | Retention time (min) | Peak area | Theoretical plates (TP) | Tailing factor (TF) |
|---|---|---|---|---|
| 1 | 2.700 | 1136.114 | 2877 | 1.441 |
| 2 | 2.700 | 1112.446 | 2966 | 1.343 |
| 3 | 2.697 | 1115.176 | 2961 | 1.455 |
| 4 | 2.707 | 1116.202 | 2976 | 1.485 |
| 5 | 2.703 | 1124.282 | 2971 | 1.485 |
| Mean | 2.7014 | 1120.844 | - | - |
| SD | 0.003782 | 9.607 | - | - |
| %RSD | 0.139984 | 0.8574 | - | - |
Table 5: Results for system suitability of Quinapril.
| Injection | Retention time (min) | Peak area | Theoretical plates (TP) | Tailing factor (TF) |
|---|---|---|---|---|
| 1 | 3.947 | 2576.974 | 2476 | 1.500 |
| 2 | 3.937 | 2535.582 | 2554 | 1.477 |
| 3 | 3.933 | 2549.337 | 2550 | 1.512 |
| 4 | 3.953 | 2538.795 | 2576 | 1.477 |
| 5 | 3.947 | 2544.742 | 2567 | 1.512 |
| Mean | 3.9434 | 2549.086 | - | - |
| SD | 0.008173 | 16.46919 | - | - |
| %RSD | 0.207261 | 0.646082 | - | - |
Table 6: Results for system suitability of Tolcapone.
| Preparations | Volume from standard stock transferred in ml | Volume made up in ml (with mobile phase) | Concentration of solution(µg /ml) | |
|---|---|---|---|---|
| QUINAPRIL | TOLCAPONE | |||
| Preparation 1 | 0.75 | 10 | 2.5 | 5 |
| Preparation 2 | 1.125 | 10 | 3.75 | 7.5 |
| Preparation 3 | 1.5 | 10 | 5 | 10 |
| Preparation 4 | 1.875 | 10 | 6.25 | 12.5 |
| Preparation 5 | 2.25 | 10 | 7.5 | 1.5 |
Table 7: Linearity preparations.
| S.No. | Conc.(µg/ml ) | Area |
|---|---|---|
| 1 | 2.5 | 495.227 |
| 2 | 3.75 | 745.541 |
| 3 | 5 | 1015.117 |
| 4 | 6.25 | 1290.46 |
| 5 | 7.5 | 1470.799 |
Table 8: Linearity of Quinapril.
| S.No. | Conc.(µg/ml ) | Area |
|---|---|---|
| 1 | 5 | 1152.124 |
| 2 | 7.5 | 1807.304 |
| 3 | 10 | 2315.072 |
| 4 | 12.5 | 2929.514 |
| 5 | 15 | 3454.098 |
Table 9: Linearity of Tolcapone.
Figure 2: Linearity graph of Tamsulosin Hydrochloride.
Figure 3: Linearity graph of Dutasteride.
Recovery
Tables 10 and 11
Observation: The percentage mean recovery of Tamsulosin Hydrochloride and Dutasteride is 100.23% and 99.47% respectively.
Precision
Observation: Test results for Tamsulosin Hydrochloride and Dutasteride are showing that the % RSD of Assay results are within limits.
| Recovery level | Accuracy Quinapril | Average % Recovery | |||
|---|---|---|---|---|---|
| Amount taken(mcg/ml) | Area | Average area | %Recovery | ||
| 50% | 2.5 | 1147.472 | 1142.193 | 101.985 | 101.54 |
| 2.5 | 1147.472 | ||||
| 2.5 | 1131.636 | ||||
| 100% | 5 | 1282.181 | 1287.862 | 103.48 | |
| 5 | 1290.460 | ||||
| 5 | 1290.945 | ||||
| 150% | 7.5 | 1391.221 | 1388.523 | 99.18 | |
| 7.5 | 1373.610 | ||||
| 7.5 | 1400.738 | ||||
Table 10: Recovery results for Quinapril.
| Recovery level | Accuracy Quinapril | Average % Recovery | |||
|---|---|---|---|---|---|
| Amount taken(mcg/ml) | Area | Average area | %Recovery | ||
| 50% | 5 | 2581.774 | 2573.486 | 102.06 | 102.81 |
| 5 | 2581.774 | ||||
| 5 | 2556.911 | ||||
| 100% | 10 | 2933.859 | 2948.693 | 105.45 | |
| 10 | 2936.438 | ||||
| 10 | 2975.781 | ||||
| 150% | 15 | 3186.091 | 3175.224 | 100.94 | |
| 15 | 3146.856 | ||||
| 15 | 3192.726 | ||||
Table 11: Recovery results for Tolcapone.
Limit of detection
Where, σ = the standard deviation of the response
S = the slope of the calibration curve
The slope S may be estimated from the calibration curve of the analyte.
LOD of Tamsulosin Hydrochloride = 0.74 µg/ml
LOD of Dutasteride = 1.29 µg/ml
Observation: The LOD for this method was found to be 0.74 µg/ml & area 33.46 for Tamsulosin Hydrochloride and 1.29 µg/ml & area 41.79 for Dutasteride.
Limit of quantification
Where,
σ = the standard deviation of the response
S = the slope of the calibration curve
The slope S may be estimated from the calibration curve of the analyte.
LOQ of Tamsulosin Hydrochloride = 2.24µg/ml
LOQ of Dutasteride =3.91 µg/ml
Observation: The LOQ for this method was found to be 2.24 µg/ml & area 101.40 for Tamsulosin Hydrochloride and 3.91 µg/ml & area 126.64 for Dutasteride.
Robustness
Observation: From the observation it was found that the system suitability parameters were within limit at all variable conditions.
RUGGEDNESS
Observation: From the observation the between two analysts Assay values not greater than 2.0%, hence the method was rugged.
| Quinapril | ||
|---|---|---|
| S.No. | Rt | Area |
| 1 | 2.660 | 1109.066 |
| 2 | 2.667 | 1110.202 |
| 3 | 2.680 | 1113.271 |
| 4 | 2.683 | 1112.450 |
| 5 | 2.680 | 1108.599 |
| 6 | 2.690 | 1109.570 |
| Avg | 2.676667 | 1110.526 |
| stdev | 0.011057 | 0.1564 |
| %RSD | 0.412278 | 0.3421 |
| Tolcapone | ||
|---|---|---|
| S.No. | Rt | Area |
| 1 | 3.890 | 2518.891 |
| 2 | 3.900 | 2515.559 |
| 3 | 3.917 | 2514.373 |
| 4 | 3.903 | 2512.866 |
| 5 | 3.913 | 2517.609 |
| 6 | 3.923 | 2519.468 |
| avg | 3.907667 | 2516.461 |
| stdev | 0.012193 | 0.4321 |
| %RSD | 0.311401 | 0.2653 |
Table 12: Method precision results for Quinapril and Tolcapone.
Discussion
A simple and selective LC method is described for the determination of Tamsulosin Hydrochloride and Dutasteride tablet dosage forms. Chromatographic separation was achieved on a C18 column using mobile phase consisting of a mixture of Phosphate buffer (KH2PO4) pH: 3.5:30 Acetonitrile: Methanol (40:30:30v/v), with detection of 223 nm. Linearity was observed in the range 19.2-44.8 µg/ml for Tamsulosin Hydrochloride (r² = 0.9961) and 24-56µg/ml for Dutasteride (r² = 0.9981) for the amount of drugs estimated by the proposed methods was in good agreement with the label claim.
The proposed methods were validated. The accuracy of the methods was assessed by recovery studies at three different levels. Recovery experiments indicated the absence of interference from commonly encountered pharmaceutical additives. The method was found to be precise as indicated by the repeatability analysis, showing % RSD less than 2. All statistical data proves validity of the methods and can be used for routine analysis of pharmaceutical dosage form.
Conclusion
From the above experimental results and parameters it was concluded that, this newly developed method for the simultaneous estimation Tamsulosin Hydrochloride and Dutasteride was found to be simple, precise, accurate and high resolution and shorter retention time makes this method more acceptable and cost effective and it can be effectively applied for routine analysis in research institutions, quality control department in meant in industries, approved testing laboratories studies in near future.
Douglas A, Skoog F, James H, Stanley RC (2007) Liquid Chromatography. In Instrumental Analysis. Cengage Learning India Pvt. Ltd, New Delhi.[ Ref ]
Skoog, Holler, Crouch (2011) Liquid Chromatography. In Instrumental Analysis, Cengage Learning India, New Delhi.[ Ref ]
Chatwal RG, Anand KS (2010) High Performance Liquid Chromatography. In Instrumental Methods Of Chemical Analysis. Himalaya Publishers, Mumbai.[ Ref ]
Sharma BK (2005) High Performance Liquid Chromatography. In Instrumental Methods Of Chemical Analysis. Goel Publishers, Meerut.[ Ref ]
Alfonso RG, Ara HDM, Glen RH, Thomas M, Nicholas GP, et al. (2000) Chromatography. In Remington: The Science and Practice of Pharmacy. Lippincott Williams & Wilkins, Philadelphia.[ Ref ]
Manoj KS, Pramod KS, Sambhu CM, Preet KK, Nitin K, et al. (2011) A perspective review on method development and validation by HPLC. International Journal of Pharmaceutical Sciences 4: 1387–1413.[ Ref ]
International Conference on Harmonization (1995) Q2A: Text on Validation of Analytical Procedures. Federal Register 60: 11260–11262.[ Ref ]
International Conference on Harmonization (1997) Q2B: Validation of Analytical Procedures: Methodology; Availability. Federal Register 62: 27463–27467.[ Ref ]
Michael Swartz E, Ira Krull S (2009) Analytical Method development. In Analytical Method Development and Validation. Marcel Dekker, Inc: New York.[ Ref ]