Journal Name: Journal of Health Science and Development
Article Type: Research
Received date: 21 October, 2021
Accepted date: 19 November, 2021
Published date: 2024-02-01
Citation: Jiang P, Liang S, Fang X, Zhai R, Liang Y, et al. (2021) Analysis of Serum Lipid Profile and Apolipoproteins in Patients with Concurrent Gallbladder Stone Disease and Type 2 Diabetes Mellitus: A Population-Based Study in China. J Health Sci Dev Vol: 4, Issue: 2 (31-37).
Copyright: 2021 Jiang P et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Gallbladder stone disease is a common disorder, and prior studies have identified an association between gallstones and abnormal serum lipids. Since patients with type 2 diabetes have higher rates of significant abnormalities in lipid metabolism, the prevalence of dyslipidemia may be higher in patients with gallstones with coexisting type 2 diabetes mellitus (T2DM) than in the general population. The purpose of this study was to compare the serum lipid and lipoprotein abnormalities between T2DM patients with gallstones and controls in China.
Methods:Serum lipid and apolipoprotein levels were retrospectively analyzed in 407 patients aged 40 years and older after they were divided into four groups: gallstones with coexisting T2DM, T2DM, gallstones, and normal. All patients had normal total blood bilirubin and were not taking lipid-lowering medications. Overall, 135 T2DM patients with gallstones were compared with three control groups comprising 102 patients with T2DM, 119 patients with gallstones, and 51 healthy individuals. Triglyceride, total cholesterol, low density lipoprotein (LDL), high density lipoprotein, very low-density lipoprotein, serum apolipoprotein AI (ApoAI), and apolipoprotein B (ApoB) levels and their ratio (ApoAI/ApoB) were measured in the four groups and compared. One-way analysis of variance and nonparametric tests (Kruskal-Wallis test) were utilized to compare the groups. Values of p<0.05 were considered statistically significant.
Results:Compared to the three control groups, T2DM patients aged 40 years and older with gallstones demonstrated significantly abnormal changes in lipids. Among the lipid parameters, LDL and ApoB levels were found to be significantly elevated in patients with coexisting gallstones and T2DM (p<0.05), while LDL levels were significantly higher in men with gallstones and T2DM (p<0.05); however, no significant differences in the parameters were found in women.
Conclusion:Serum lipid abnormalities appear to be common in patients over 40 years of age with gallstones and T2DM. LDL and ApoB levels were significantly different in patients over 40 years of age with concurrent gallstone disease and T2DM, with men having significantly higher LDL levels. However, the effect of serum lipid concentration on the high prevalence of gallstones in women over 40 years of age with concurrent gallstones and T2DM could not be determined.
Keywords:
Serum lipids, Apolipoproteins, Gallstone disease, Type 2 diabetes mellitus.
Abbreviations
HDL-high-density lipoprotein; LDL-low-density lipoprotein; TC-total cholesterol; TG-triglyceride; ApoAIapolipoprotein AI ; ApoB-apolipoprotein B; N-normal group; D-diabetic group; G-gallstone group; GD-diabetes plus gallstone group; GSD-gallbladder stone diseases; T2DM-type 2 diabetes mellitus; ANOVA-one-way analysis of variance; SD-standard deviation; IQR-interquartile range.
Background: Gallbladder stone disease is a common disorder, and prior studies have identified an association between gallstones and abnormal serum lipids. Since patients with type 2 diabetes have higher rates of significant abnormalities in lipid metabolism, the prevalence of dyslipidemia may be higher in patients with gallstones with coexisting type 2 diabetes mellitus (T2DM) than in the general population. The purpose of this study was to compare the serum lipid and lipoprotein abnormalities between T2DM patients with gallstones and controls in China.
Methods:Serum lipid and apolipoprotein levels were retrospectively analyzed in 407 patients aged 40 years and older after they were divided into four groups: gallstones with coexisting T2DM, T2DM, gallstones, and normal. All patients had normal total blood bilirubin and were not taking lipid-lowering medications. Overall, 135 T2DM patients with gallstones were compared with three control groups comprising 102 patients with T2DM, 119 patients with gallstones, and 51 healthy individuals. Triglyceride, total cholesterol, low density lipoprotein (LDL), high density lipoprotein, very low-density lipoprotein, serum apolipoprotein AI (ApoAI), and apolipoprotein B (ApoB) levels and their ratio (ApoAI/ApoB) were measured in the four groups and compared. One-way analysis of variance and nonparametric tests (Kruskal-Wallis test) were utilized to compare the groups. Values of p<0.05 were considered statistically significant.
Results:Compared to the three control groups, T2DM patients aged 40 years and older with gallstones demonstrated significantly abnormal changes in lipids. Among the lipid parameters, LDL and ApoB levels were found to be significantly elevated in patients with coexisting gallstones and T2DM (p<0.05), while LDL levels were significantly higher in men with gallstones and T2DM (p<0.05); however, no significant differences in the parameters were found in women.
Conclusion:Serum lipid abnormalities appear to be common in patients over 40 years of age with gallstones and T2DM. LDL and ApoB levels were significantly different in patients over 40 years of age with concurrent gallstone disease and T2DM, with men having significantly higher LDL levels. However, the effect of serum lipid concentration on the high prevalence of gallstones in women over 40 years of age with concurrent gallstones and T2DM could not be determined.
Keywords:
Serum lipids, Apolipoproteins, Gallstone disease, Type 2 diabetes mellitus.
Abbreviations
HDL-high-density lipoprotein; LDL-low-density lipoprotein; TC-total cholesterol; TG-triglyceride; ApoAIapolipoprotein AI ; ApoB-apolipoprotein B; N-normal group; D-diabetic group; G-gallstone group; GD-diabetes plus gallstone group; GSD-gallbladder stone diseases; T2DM-type 2 diabetes mellitus; ANOVA-one-way analysis of variance; SD-standard deviation; IQR-interquartile range.
Introduction
Gallstone disease (GSD) is one of the most common gastrointestinal disorders worldwide, which imposes a substantial economic burden on health care systems with a reported incidence rate of 15% [1]. Possible risk factors include sex, age, obesity, diabetes, dyslipidemia, rapid appetite loss, hepatitis C, and cirrhosis, with diabetes being strongly associated with the incidence of GSD [2- 8]. Type 2 diabetes mellitus (T2DM) and prediabetes are commonly associated with dyslipidemia and hypertension [9]. The similar risk factors for T2DM and gallstones may be responsible for the increased risk of gallstones in patients with T2DM. A growing number of studies have suggested that lipid disorder is a common risk factor for both GSD and T2DM, which can also increase the risk of coronary heart disease and stroke [10-13]. Although retrospective clinical studies have shown that changes in the lipid profile occur not only in patients with cholelithiasis but also in patients who undergo laparoscopic cholecystectomy, few studies have compared the serum lipid abnormalities in patients with concomitant gallstones and T2DM with healthy control [14-16]. The aim of this study was to analyze the serum lipid profile and apolipoprotein levels in Chinese patients with concurrent gallstones and T2DM aged 40 years and above.
Methods
This retrospective study was conducted in the Department of General Surgery of Lu’An People’s Hospital affiliated to Anhui Medical University from July 2016 to June 2018. The study was approved by the Ethics Committee of the People’s Hospital of Lu’An. Written informed consent was obtained from the enrolled patients or their guardians for the use of test results and personal information in this study. A total of 407 individuals were divided into four groups: T2DM and gallstones, gallstones only, T2DM only, and healthy controls. All participants were 40 years or older and underwent serum lipid profile and apolipoprotein analysis from July 2016 to June 2018. The inclusion criteria were age ≥40 years, residency in China, normal total bilirubin concentration, and no intake of lipid-lowering drugs. Patients with alcoholic cirrhosis, intrahepatic stones, renal failure, nephrotic syndrome, pancreatitis, heart failure, morbid obesity, hypothyroidism, sickle cell disease, hemoglobinopathy, and pregnancy, as well as patients undergoing cholecystectomy or taking antilipemic drugs were excluded from this study.
Patients with T2DM and gallstones were classified into the diabetic stones group, with ages ranging from 40 to 84 years (mean age 58.92±9.59 years); patients with T2DM without gallstones were classified into the diabetic group, with ages ranging from 40 to 76 years (mean age 56.74 ± 9.35 years); patients with gallstones without T2DM were classified into the gallstone group, with ages ranging from 40 to 91 years (mean age 58.03±12.04 years); and in the healthy group, ages ranged from 40 to 84 years with a mean age of 57.21 ± 10.40 years. The diabetic stones group was abbreviated as the GD group and used as the case group. The diabetic group, gallstone group, and normal group were abbreviated as the D group, G group, and N group, respectively, and were all designated as the control group.
Patients in the GD and G groups visited the hospital to undergo cholecystectomy. Cholesterol gallstones were identified by the visual inspection of a representative section of the gallstones or, if required, by enzymatic cholesterol analysis. Fasting blood samples were obtained from all patients by venipuncture under aseptic conditions before starting the treatment. The fasting serum lipid profile sample was collected along with the samples for other preoperative investigations. Blood samples collected from the cases and controls were used to measure creatinine, uric acid, fasting glycemia, the serum lipid profile, and apolipoproteins. These measurements were performed using the Beckman Coulter AU5800 automated biochemical analyzer (Beckman Coulter, Inc., Brea, CA, USA). The serum lipid profile and apolipoprotein parameters measured included total cholesterol (TC), triglycerides (TG), highdensity lipoproteins (HDL), low-density lipoproteins (LDL), very-low-density lipoproteins (VLDL) as well as serum apolipoprotein AI (ApoAI), apolipoprotein B (ApoB), and their ratio (ApoAI/ApoB).
Statistical analysis
The statistical analyses were performed using SPSS software, version 25.0 for Windows (SPSS, Chicago, IL), and figures were generated using GraphPad Prism software (v 5.01; GraphPad Software, La Jolla, CA, USA). The continuous variables were tested for normality by the Shapiro-Wilk normality test. A one-way analysis of variance (ANOVA) was used to compare the age differences among the groups, while the sex differences between the groups were assessed using chi-square statistics. If numerical variables were normally distributed, they are expressed as means ± standard deviation (SD). The quantitative data were compared using one-way ANOVA; for multiple comparisons, the Bonferroni’s test was used when the variance was homogeneous, while the Tamhane’s T2 test was used when the variance was not homogeneous. If the numerical variables were not normally distributed, they were expressed as medians (interquartile range [IQR]), and the Kruskal-Wallis test with Bonferroni correction was used for multiple comparisons. Values of p<0.05 were considered statistically significant.
Results
The mean ages of the GD group, G group, D group, and N group were 58.92 ± 9.59 years, 58.03 ± 12.04 years, 56.74 ± 9.35 years, and 57.21 ± 10.40 years, respectively. The GD group consisted of 86 women and 49 men, with a female to male ratio of 1.76:1; the N group comprised 28 women and 23 men, with a ratio of 1.22:1; the D group comprised 36 women and 66 men with a ratio of 0.55:1; and the G group comprised 84 women and 35 men, with a ratio of 2.4:1. Data regarding age, body weight, creatinine, uric acid, fasting glycemia, and sex are presented in table 1. No significant difference was observed in the sex distribution among the four groups (P=0.36). Although the sex ratios differed significantly, the comparisons among the groups were performed separately for each sex; therefore, the parameter of sex did not affect the results. Between the GD and D groups, there were no statistically significant differences in creatinine (67.65 ± 20.42 vs 64.14 ± 15.50 μmol/L, P=0.58) and fasting glycemia (8.54 ± 2.98 vs 10.79 ± 21.92 mmol/L, P=0.89) (Supplementary Table 1), and creatinine and glycemic values were higher in the D and GD groups than those surveyed in the N and G groups (Table 1). In terms of body weight and uric acid values, none of these variables were assessed too differently among the four groups (P>0.05).
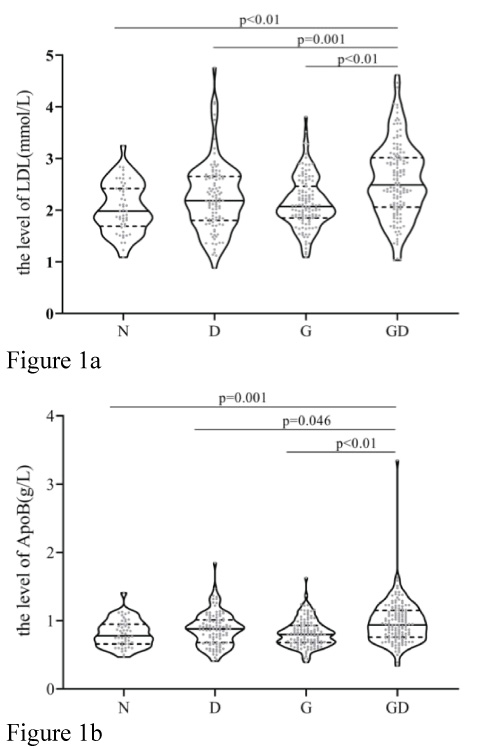
The mean serum lipid and apolipoprotein concentrations, including TC, TG, LDL, HDL, VLDL, APOAI, ApoB, and APOAI/ APOB, in patients aged 40 years and above are shown in table 2. Comparison of the total serum lipid profile showed that the mean serum levels of TC, TG, and VLDL were higher in the GD group than in the other three groups, although the difference was not significant (P>0.05). However, LDL levels and ApoB levels showed significant differences (P<0.05) (Table 3). The mean levels of ApoAI/B were lower in patients with T2DM and gallstones, but the difference was not significant. The differences in the serum levels of HDL and ApoB in patients with gallstones and T2DM were not significant (P>0.05) compared to other groups (Table 2). The extracted data are represented by violin plots, with quartiles represented by dotted lines and medians represented by full lines in figure 1. This analysis revealed significant differences in the levels of LDL (Figure 1a) and apolipoprotein (Figure 1b), with the GD group demonstrating higher levels than the other three groups.
Table 1:Distribution of age, body weight, creatinine, uric acid, fasting glycemia, and sex in patients >40 years in the case and three control groups
| Physical Parameter | N | D | G | GD | P |
|---|---|---|---|---|---|
| Age (years) | 57.21 ± 10.40 | 56.74 ± 9.35 | 58.03 ± 12.04 | 58.92 ± 9.59 | 0.36* |
| body weight (kg) | 64.22 ± 9.56 | 66.13 ± 11.87 | 63.8 ± 10.32 | 66.85 ± 10.38 | 0.11* |
| Creatinine (μmol/L) | 57.94 ± 14.13 | 64.14 ± 15.50 | 58.07 ± 13.21 | 67.65 ± 20.42 | <0.001* |
| uric acid (μmol/L) | 285.95 ± 72.00 | 294.01 ± 91.58 | 287.77 ± 71.25 | 298.69 ± 81.98 | 0.66* |
| fasting glycemia (mmol/L) | 5.36 ± 0.73 | 10.79 ± 21.92 | 5.38 ± 0.90 | 8.54 ± 2.98 | 0.001 |
| Sex (n,%) | |||||
| Women | 28(12.00%) | 36(15.4%) | 84(35.9%) | 86(36.8%) | <0.01** |
| Men | 23(13.3%) | 66(38.2%) | 35(20.2%) | 49(28.3%) | |
| The ages, body weight, creatinine, uric acid, and fasting glycemia are shown as means ± SD. Abbreviations: SD, standard deviation; N, normal group; D, diabetic group; G, gallstone group; GD, diabetes plus gallstone group; *One-way analysis of variance (ANOVA), ** chi-square statistics; Significance was accepted at p values <0.05. Units of creatinine and uric acid are μmol/L; units of fasting glycemia are mmol/L; units of body weight are kg. | |||||
Table 2:Medians and interquartile ranges of lipid profile and apolipoprotein levels in the case and three control groups.
| Physical Parameter | N | D | G | GD | P* |
|---|---|---|---|---|---|
| TC | 3.98(3.71~4.99) | 4.34(3.56~4.88) | 4.49(4~4.95) | 4.66(3.91~5.34) | 0.014 |
| TG | 1.03(0.86~1.39) | 1.23(0.87~1.63) | 1.19(0.91~1.78) | 1.38(1.05~1.94) | <0.001 |
| HDL | 1.26(1.06~1.42) | 1.23(0.99~1.46) | 1.2(0.98~1.41) | 1.27(1.07~1.51) | 0.246 |
| LDL | 1.98(1.69~2.42) | 2.19(1.8~2.659) | 2.07(1.85~2.46) | 2.49(2.06~3.02) | <0.001 |
| VLDL | 0.49(0.39~0.65) | 0.55(0.39~0.73) | 0.54(0.41~0.8) | 0.62(0.47~0.87) | 0.001 |
| ApoAI | 1.25(1.14~1.34) | 1.19(0.99~1.35) | 1.23(1.07~1.38) | 1.22(1.03~1.42) | 0.257 |
| ApoB | 0.78(0.66~0.95) | 0.88(0.68~1.01) | 0.8(0.68~0.93) | 0.94(0.76~1.15) | <0.001 |
| ApoAI/ApoB | 1.6(1.33~1.89) | 1.34(1.09~1.82) | 1.49(1.24~1.86) | 1.27(1.08~1.53) | <0.001 |
| All the results are expressed as medians and interquartile ranges as the distribution was not normal. The data were analyzed with the Kruskal–Wallis non-parametric test and Shapiro-Wilk test to check for normal distribution. Significance was accepted at p values <0.05. *Kruskal–Wallis non-parametric test. Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; TC, total cholesterol; TG, triglyceride; ApoAI, apolipoprotein AI ; ApoB, apolipoprotein B; N, normal group; D, diabetic group; G, gallstone group; GD, diabetes plus gallstone group. Units of TC, TG, HDL, and LDL are mmol/L; units of Apo A1 and Apo B are g/L. | |||||
Table 3:Multiple comparisons of LDL and ApoB levels in patients over 40 years of age among the case and three control groups.
| Physical Parameter | Sample 1-Sample 2 | Test Statistic | Std. Error | Std. Test Statistic | P | Pa |
|---|---|---|---|---|---|---|
| LDL | GD-N | -92.451 | 19.334 | -4.782 | <0.001 | <0.01 |
| GD-G | -75.041 | 14.791 | -5.073 | <0.001 | <0.01 | |
| GD-D | -58.657 | 15.432 | -3.801 | <0.001 | <0.01 | |
| ApoB | GD-N | -71.026 | 19.332 | -3.674 | <0.001 | 0.001 |
| GD-G | -69.661 | 14.789 | -4.71 | <0.001 | <0.01 | |
| GD-D | -41.169 | 15.431 | -2.668 | 0.008 | 0.046 | |
| For statistical comparisons between the groups, the Kruskal-Wallis H test followed by testing for multiple comparisons was performed. Significance was accepted at pa values <0.05. Abbreviations: LDL, low-density lipoprotein; ApoB, apolipoprotein B. aSignificant values were adjusted by the Bonferroni correction for multiple tests. | ||||||
Table 4:Median and mean values of the lipid profile and apolipoprotein levels in the case and three control groups.
| Physical Parameter | N | D | G | GD | P |
|---|---|---|---|---|---|
| TC | 3.77(3.65~4.91) | 4.32(3.43~4.82) | 4.46(3.73~4.93) | 4.37(3.57~5.3) | 0.398** |
| TG | 1.08(0.76~1.39) | 1.22(0.76~1.63) | 1.19(0.84~1.94) | 1.19(0.84~1.94) | 0.046** |
| HDL | 1.22(1~1.41) | 1.24(0.97~1.4) | 1.09(0.85~1.26) | 1.19(0.94~1.35) | 0.13** |
| LDL | 1.96 ± 0.5 | 2.16±0.63 | 2.11±0.48 | 2.54 ± 0.75 | 0.002* |
| VLDL | 0.5(0.34~0.68) | 0.55(0.34~0.73) | 0.54(0.38~0.87) | 0.64(0.43~0.9) | 0.109* |
| ApoAI | 1.21(1.08~1.32) | 1.15(0.97~1.28) | 1.07(1~1.27) | 1.13(0.93~1.33) | 0.169** |
| ApoB | 0.75(0.6~0.9) | 0.86(0.66~0.98) | 0.79(0.69~0.89) | 0.91(0.76~1.12) | 0.015** |
| ApoAI/ApoB | 1.66(1.33~1.94) | 1.37(1.09~1.87) | 1.34(1.2~1.61) | 1.19(0.98~1.46) | 0.002** |
| LDL concentrations are expressed as means ± SD; concentrations of TC, TG, HDL, VLDL, ApoAI, ApoB, and ratio of ApoAI/ApoB are expressed as medians and interquartile ranges. * One-way analysis of variance (ANOVA); ** Kruskal–Wallis non-parametric test; Significance was accepted at p values <0.05. Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; TC, total cholesterol; TG, triglyceride; ApoAI, apolipoprotein AI ; ApoB, apolipoprotein B; N, normal group; D, diabetic group; G, gallstone group; GD, diabetes plus gallstone group. | |||||
Table 5:Multiple comparisons of LDL levels in men aged over 40 years among the case and three control groups.
| Physical Parameter | Sample 1(I)-Sample 2(J) | Mean Difference (I-J) | Std. Error | 95% Confidence Interval | P | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| LDL | GD-N | 0.58 | 0.15 | 0.18 | 0.99 | 0.001 |
| GD-G | 0.38 | 0.38 | 0.02 | 0.74 | 0.030 | |
| GD-D | 0.43 | 0.13 | 0.07 | 0.80 | 0.011 | |
| Concentrations of LDL are expressed as the means ± SD, and between-group differences were assessed by one-way ANOVA followed by the Tamhane’s T2 test for multiple comparisons (homogeneity of variance was not equal). Significance was accepted at p values < 0.05. Abbreviations: LDL, low-density lipoprotein; N, normal group; D, diabetic group; G, gallstone group; GD, diabetes plus gallstone group. | ||||||
Figure 1:The median and interquartile range(IQR) of the LDL(Figure 1a) and ApoB (Figure 1b) levels for the GD group and three control groups(N, D, G groups), the median, upper and lower quartiles, the range were shown as a violin plot. Abbreviations: LDL, low-density lipoprotein; ApoB, apolipoprotein B; N, normal group; D, diabetic group; G, gallstone group; GD, diabetes plus gallstone group.
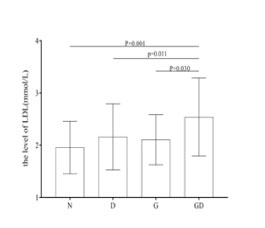
Figure 2:The mean and standard deviation(SD) of the LDL level for the GD group and three control groups(N, D, G groups), the mean and SD were shown as a bar chart. Abbreviations: LDL, low-density lipoprotein; N, normal group; D, diabetic group; G, gallstone group; GD, diabetes plus gallstone group.
The mean serum TC and TG were higher in GSD patients with T2DM than in controls, although the difference was not significant. Several studies have reported higher serum cholesterol and triglyceride levels in patients with gallstones compared to healthy controls [16]. However, it was found that the correlation between high levels of cholesterol and triglycerides and gallstones was not significant [24]. Moreover, the relationship between gallstone formation and elevated serum cholesterol and triglyceride levels has been found to be controversial in the literature. We observed higher concentrations of LDL and VLDL in the GD group than in the three control groups. Notably, the differences in the LDL levels between the GD group and control groups were significant, but the differences in VLDL levels were not significant. Additionally, some studies have shown no association between low HDL levels and gallstone disease [25]. Since LDL is the main protein that transports cholesterol, and most cholesterol in the blood is present in LDL, elevated LDL levels lead to increased transport of peripheral cholesterol into the liver, which may lead to cholesterol supersaturation. Furthermore, previous studies have described a positive correlation between serum LDL levels and gallstones [26].
The most prominent application of lipoprotein measurements in clinical biochemistry is as markers of cardiovascular risk [27]. ApoAI and ApoB are the major apolipoproteins for HDL and LDL, respectively. It has been reported that serum apolipoprotein levels are likely to be more sensitive than serum lipid levels in distinguishing patients with gallstones from those without stones, despite the lack of changes in the lipid levels [28]. In the present study, we found increased concentrations of serum ApoB in the GD group, and the difference in the ApoB levels was significant. The main role of ApoAI and ApoB in the pathogenesis of gallbladder disease may be related to the transport of cholesterol and nucleation of bile cholesterol. Our results suggest that the serum concentrations of LDL and HDL and the respective ApoB and ApoAI levels follow a similar pattern of association, and demonstrate samedirectional changes in terms of the serum concentration, which is characterized by increased LDL and ApoB levels along with reduced HDL and ApoAI levels. This finding is in agreement with other studies [29]. Meanwhile, we observed higher concentrations of ApoB in the GD group than in the control group, and the difference in the ApoB concentration was significant. This finding further illustrates the significant role of serum LDL and ApoB concentrations in gallstone formation and the occurrence of diabetes among patients with gallstone disease.
The results of the present study also revealed that in men aged 40 years and above, the median concentrations of LDL and ApoB were higher in gallstone patients with T2DM than in patients in the other three groups, and the difference in the LDL concentration was significant. The prevalence of dyslipidemia depends on the sex, with men having a higher incidence. Lifestyle may be considered a possible cause for such an observation, as there may be more risk factors for dyslipidemia among men due to lifestyle factors such as smoking, drinking alcohol, and so on. However, no association between serum lipids and high incidence of gallstones in women was found in the present study. There could be more complex factors that would influence the changes in the serum lipid profile and apolipoprotein levels in women. Studies carried out by Gul et al. and Halgaonkar et al. have shown that reproductive hormones play a role in the pathogenesis of gallstones in women, in addition to the race and eating habits. Perhaps, further studies are needed to verify our conjectures [30,31].
Limitations of the Study
The present study has several limitations. First, the study was retrospective and was conducted in a single center. Second, the records of height values in the study were incomplete, preventing further analysis of body mass index, which is also a limitation of the study.
Conclusion
Serum lipid abnormalities appear to be common in patients over 40 years of age with gallstones and T2DM. This study showed that LDL and ApoB levels are significantly higher in patients aged >40 years with gallstones and concurrent T2DM, with men having significantly higher LDL levels than women in each of the three control groups. However, the effect of serum lipid concentration on the high prevalence of gallstones in women over 40 years of age with gallstones and T2DM could not be determined. From the study, the disorder of lipid metabolism may be a contributing factor among the many causes of T2DM with gallbladder stones. Serum LDL and ApoB levels are sensitive indicators and thus can be helpful in the prevention of T2DM combined with gallstones.
Acknowledgments
We thank our staff for fruitful discussions and clerical support.
Availability of Data and Materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ Contribution
Pin Jiang and Rong-rong Zhai collected and analyzed all the included data. Song Liang designed this study and drafted the manuscript. All of the authors have read and approved the final version of the manuscript.
Ethics Approval and Consent to Participate
The present study was approved by the Ethics Committee of Lu’an People’s Hospital. Informed consent was obtained from all the participants.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Portincasa P, Di Ciaula A, de Bari O, Garruti G, Palmieri VO, et al. (2016) Management of gallstones and its related complications. Expert Rev Gastroenterol Hepatol 10: 93-112. [ Ref ]
Hung SC, Liao KF, Lai SW, Li CI, Chen WC (2011) Risk factors associated with symptomatic cholelithiasis in Taiwan: a population-based study. BMC Gastroenterol 11: 111. [ Ref ]
Li X, Gao P (2018) Hepatitis C Virus Infection Increases Risk of Gallstone Disease in Elderly Chinese Patients with Chronic Liver Disease. Sci Rep 8: 4636. [ Ref ]
Li X, Wang Z, Wang L, Pan M, Gao P (2017) Liver cirrhosis: a risk factor for gallstone disease in chronic hepatitis C patients in China. Medicine (Baltimore) 96: e7427. [ Ref ]
Portincasa P, Moschetta A, Palasciano G (2006) Cholesterol gallstone disease. Lancet 368: 230-239. [ Ref ]
Lioudaki E, Ganotakis ES, Mikhailidis DP (2011) Lipid lowering drugs and gallstones: a therapeutic option? Curr Pharm Des 17: 3622-3631. [ Ref ]
Shebl FM, Andreotti G, Meyer TE, Gao YT, Rashid A, et al (2011) Metabolic syndrome and insulin resistance in relation to biliary tract cancer and stone risks: a population-based study in Shanghai, China. Br J Cancer 105: 1424-1429. [ Ref ]
Nakeeb A, Comuzzie AG, Al-Azzawi H, Sonnenberg GE, Kissebah AH, et al. (2006) Insulin resistance causes human gallbladder dysmotility. J Gastrointest Surg 10: 940-948. [ Ref ]
Xu H, He L, Liu C, Tang L, Xu Y, et al (2016) LncRNA NONRATT021972 siRNA attenuates P2X7 receptor expression and inflammatory cytokine production induced by combined high glucose and free fatty acids in PC12 cells. Purinergic Signal 12: 259-268. [ Ref ]
Laakso M, Suhonen M, Julkunen R, Pyörälä K (1990) Plasma insulin, serum lipids and lipoproteins in gall stone disease in non-insulin dependent diabetic subjects: a case control study. Gut 31: 344-347. [ Ref ]
Burcelin R, Serino M, Chabo C, Blasco-Baque V, Amar J (2011) Gut microbiota and diabetes: from pathogenesis to therapeutic perspective. Acta Diabetol 48: 257-273. [ Ref ]
Lv J, Qi L, Yu C, Guo Y, Bian Z, et al (2015) Gallstone Disease and the Risk of Ischemic Heart Disease. Arterioscler Thromb Vasc Biol 35: 2232- 2237. [ Ref ]
Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, et al. (2012) Host-gut microbiota metabolic interactions. Science 336: 1262-1267. [ Ref ]
Batajoo H, Hazra NK (2013) Analysis of serum lipid profile in cholelithiasis patients. J Nepal Health Res Counc 11: 53-55. [ Ref ]
Gill GS, Gupta K (2017) Pre- and Post-operative Comparative Analysis of Serum Lipid Profile in Patients with Cholelithiasis. Int J Appl Basic Med Res 7: 186-188. [ Ref ]
Hayat S, Hassan Z, Changazi SH, Zahra A, Noman M, et al. (2019) Comparative analysis of serum lipid profiles in patients with and without gallstones: A prospective cross-sectional study. Ann Med Surg 42: 11-13. [ Ref ]
Weerakoon HT, Ranasinghe S, Navaratne A, Sivakanesan R, Galketiya KB, (2014) Serum lipid concentrations in patients with cholesterol and pigment gallstones. BMC Res Notes 7: 548. [ Ref ]
Kurtul N, Pençe S, Kocoglu H, Aksoy H, Capan Y (2002) Serum lipid and lipoproteins in gallstone patients. Acta Medica (Hradec Kralove) 45: 79- 81. [ Ref ]
Di Ciaula A, Wang DQ, Portincasa P (2018) An update on the pathogenesis of cholesterol gallstone disease. Curr Opin Gastroenterol 34: 71-80. [ Ref ]
Wang W, Li N (2014) The association of gallstone disease and diabetes mellitus. A meta-analysis. Saudi Med J 35: 1005-1012. [ Ref ]
Chen LY, Qiao QH, Zhang SC, Chen YH, Chao GQ, et al. (2012) Metabolic syndrome and gallstone disease. World J Gastroenterol 18: 4215-4220. [ Ref ]
Zhu Q, Sun X, Ji X, Zhu L, Xu J, et al. (2016) The association between gallstones and metabolic syndrome in urban Han Chinese: a longitudinal cohort study. Sci Rep 6: 29937. [ Ref ]
Liang D, Fan G (2020) Social Support and User Characteristics in Online Diabetes Communities: An In-Depth Survey of a Large-Scale Chinese Population. Int J Environ Res Public Health 17: 2806. [ Ref ]
Ömer C, Güneri MC (2012) Lipid profiles of patients with gallstones. Turkish Journal of Family Practice 16: 123-126. [ Ref ]
Al-Saadi NH, Al-Ardhi SA (2012) Biochemical and demographical study of lipid profile in sera of patients with gallstone. Iraqi Journal of Science 53: 760-768. [ Ref ]
Gul H, Ayub M, Akhtar A (2016) Mean serum calcium and lipid profile in patients with gallstone disease in southern Punjab. Pak J Med Health Sci 1: 548-551. [ Ref ]
Dominiczak MH, Caslake MJ (2011) Apolipoproteins: metabolic role and clinical biochemistry applications. Ann Clin Biochem 48: 498-515. [ Ref ]
Zhao JC, Xiao LJ, Zhu H, Shu Y, Cheng NS (1998) Changes of lipid metabolism in plasma, liver and bile during cholesterol gallstone formation in rabbit model. World J Gastroenterol 4: 337-339. [ Ref ]
Morán S, Duque-López MX, Salmerón-Castro J, Rodríguez-Leal G, Martínez-Salgado H, et al. (2003) Association between serum concentration of apolipoproteins A-I and B with gallbladder disease. Arch Med Res 34: 194-199. [ Ref ]
Agha RA, Borrelli MR, Vella-Baldacchino M, Thavayogan R, Orgill DP (2017) The STROCSS statement: Strengthening the Reporting of Cohort Studies in Surgery. Int J Surg 46: 198-202. [ Ref ]
Halgaonkar P, Verma R, Bhadre R, Unadkat P, Vaja C, et al. (2016) Study to establish the clinical correlation between chemical constituents of gallstones and serum biochemical parameters. International Journal of Scientific Study 4: 97-102. [ Ref ]