Journal Name: Journal of Health Science and Development
Article Type: Research
Received date: 13 October, 2021
Accepted date: 26 November, 2021
Published date: 2024-02-01
Citation: Gieseler F, Rose G, Rades D, Dunst J, Bubnoff N, Luebbe A (2021) EORTC Quality of Life Questionnaire with added Subjective Weighting for Improving Tailored Treatment and Therapy Monitoring in Oncology. J Health Sci Dev Vol: 4, Issue: 2 (38-42).
Copyright: © 2021 Gieseler F et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: It is good medical practice to use validated questionnaires to compare different treatment options in oncological therapy studies. Over the course of the past few years, it has been suggested that patient-reported outcome measures (PROMs) could also be of value in monitoring individual treatment schemes, especially in the palliative care setting, where quality of life (QoL) is of primary importance. Although the EORTC-QLQ-C30 comprises a set of personal questions, patients are not asked about the subjectively assessed functional impairment associated with the symptom in their individual life situation.
Methods:We examined whether the results of the EORTC QLQ C-30, one of the most frequently used QoL questionnaires, would be different if the subjective interpretation of symptoms assessed on a function scale, such as physical functioning, are added to the scores. For each of the five functional scales of the EORTC-QLQ C30 the patients were asked to provide a subjective weighting, e.g. “How would you currently rate your physical functioning on a scale from 1 to 5?”. A total of 95 answers from 13 patients were evaluated in part at several time points of their therapy. All patients included in this study had various cancers and were receiving only symptomatic but not curative radiation therapy (cerebral or bone).
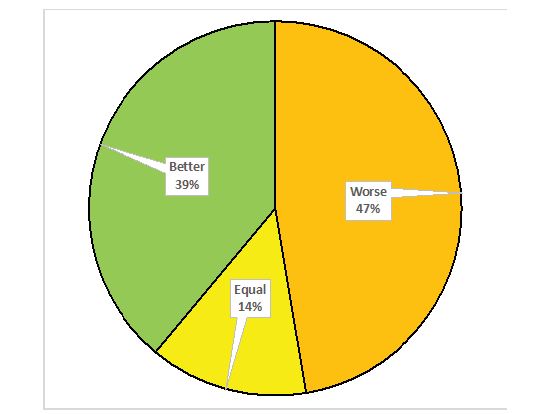
Results:By adding the weighting question 86% of answers changed, with 39% of the answers being more positive and 47 % more negative when comparing EORTC QLQ-C30 results and subjective rating.
Conclusion:The above results show that the addition of the question of functional impairment resulting from a symptom might enable the use of standard questionnaires like the EORTC QLQ-C30 as an instrument for individual therapy management. Further investigation into how the standard questionnaire needs to be adapted is clearly needed und justified.
Keywords:
EORTC Quality, Oncology,
List of Abbreviations:
PROMs: patient-reported outcome measures; QoL: Quality of life; QLQs: Quality of life questionnaires; MSAS: Memorial Symptom Assessment Scale.
Background: It is good medical practice to use validated questionnaires to compare different treatment options in oncological therapy studies. Over the course of the past few years, it has been suggested that patient-reported outcome measures (PROMs) could also be of value in monitoring individual treatment schemes, especially in the palliative care setting, where quality of life (QoL) is of primary importance. Although the EORTC-QLQ-C30 comprises a set of personal questions, patients are not asked about the subjectively assessed functional impairment associated with the symptom in their individual life situation.
Methods:We examined whether the results of the EORTC QLQ C-30, one of the most frequently used QoL questionnaires, would be different if the subjective interpretation of symptoms assessed on a function scale, such as physical functioning, are added to the scores. For each of the five functional scales of the EORTC-QLQ C30 the patients were asked to provide a subjective weighting, e.g. “How would you currently rate your physical functioning on a scale from 1 to 5?”. A total of 95 answers from 13 patients were evaluated in part at several time points of their therapy. All patients included in this study had various cancers and were receiving only symptomatic but not curative radiation therapy (cerebral or bone).
Results:By adding the weighting question 86% of answers changed, with 39% of the answers being more positive and 47 % more negative when comparing EORTC QLQ-C30 results and subjective rating.
Conclusion:The above results show that the addition of the question of functional impairment resulting from a symptom might enable the use of standard questionnaires like the EORTC QLQ-C30 as an instrument for individual therapy management. Further investigation into how the standard questionnaire needs to be adapted is clearly needed und justified.
Keywords:
EORTC Quality, Oncology,
List of Abbreviations:
PROMs: patient-reported outcome measures; QoL: Quality of life; QLQs: Quality of life questionnaires; MSAS: Memorial Symptom Assessment Scale.
Introduction
The evaluation and documentation of QoL of patients using PROMS has become standard in modern clinical research, especially in oncology [1,2]. PROMs are self-completed questionnaires that focus on the patients’ perception of their current state of health at a single time point that includes their functional ability, mental and emotional status as well as their QoL [3]. Due to the individual definition of QoL, it is a complex task to develop questionnaires that deliver results that are comparable throughout different life situations such as social, financial, or familial [4,5]. Several questionnaires have been developed and evaluated over the course of the past years [2,5,6].
PROM questionnaires can be described as either generic, condition-specific, or disease-specific [3,7].
Disease-specific PROMs might have a higher validity for the given disease (e.g. cancer sub-types) because they focus on particular concerns in specific cancers.
Condition-specific questionnaires focus on specific groups of patients with a given disease, e.g. older patients or patients with specific co-morbidities.
Disease- and condition specific PROMs offer greater validity when compared to generic PROMs; often, generic and disease specific PROMs are used in combination to obtain a complementary dataset [7].
The original purpose behind the development of quality of life questionnaires (QLQs) was their use as one end point in cancer clinical studies [8]. Individual interpretations by the patients did not play a major role in the recording. Symptoms must be recorded as accurately as possible (“shortness of breath, grade 2”) without subjective interpretation by the patient (“horrible symptom”) in order to achieve a factual description and enable comparison of different treatment plans. However, when two different treatments reveal comparable response rates, or when maintaining QoL is an important goal of treatment (e.g. in palliative oncology), the results of PROMs may help physicians decide on the therapy that is more appropriate to their patients in their individual situation. Besides their use as one end point in clinical therapy studies, the use of PROMs has recently been recommended for monitoring therapy- and side-effects in individual patients [9-11]. However, successful repurposing of PROMS for such monitoring requires input from patients about their subjective experiences during the course of cancer therapy.
Methods and Methods
We compared the results of the classical interpretation of the EORTC QLQ-C30 questionnaire with that of the questionnaire when an individual weighting of the symptom was added. As subjective weighting, the question patients were asked was: How would you currently rate your <functional scale > on a scale from 1 to 5? (1 = very well, no disturbance and 5 = very badly, extreme disturbance). We chose the same five functioning scales that the EORTCQLQ- C30 uses, which are physical, emotional, cognitive, role and social functioning.
In the QLQ-C30, multiple questions (= items) contribute towards one functioning scale. The answers to these items are added together to determine a raw score, be-fore using linear transformation to reach a value between 0 and 100. “A high score for a functioning scale represents a high / healthy level of functioning”. For details on how the final scores are calculated, please refer to the EORTC scoring manual (https://qol.eortc.org).
To enable a comparison of the EORTC QLQ-C30 results and the results of the personalized questionnaire, we transformed the answers between 1 and 5 given by the patients into values between 0 and 100 (each step between 1-5 being 20%). The comparison between the QLQ-C30 values and the subjective weighting question values were achieved by subtracting the scores for each scale. In order to visualize larger and smaller differences, and whether the individual weighting was more positive or more negative than the score given by the QLQ-C30, we divided the responses in groups depending on the degree of deviation (see methods section, table 1). The quantitative analysis for the distinctive functioning using code numbers ranging from +4 (much better) to -4 (much worse) are shown in table 2.
A total of 95 answers from 13 patients with different cancers were evaluated in part at several time points during the course of their therapy. All patients underwent symptomatic radiation therapy (cerebral or bone) and not therapy with a curative intent (for patient characteristics see table 3). The patients were given the questionnaires at the University Department of Radiotherapy in Kiel or Luebeck. They were asked to complete the original EORTC QLQ C-30 questionnaire (https://qol.eortc.org) as well as the additional weighting question on a tablet personal computer (iPad) for documentation. After log-in and pseudonymization of the patients’ data, the answers given were stored by KAIKU Health, Finland (https://kaikuhealth.com). Results were then analyzed using Microsoft Excel for Mac (ver. 16.16.17). The scientific approach and data management was approved by the ethics committee of the University of Luebeck (AZ 18- 287).
When comparing the individual weighting to the interpretation of the standard tools, there was a difference in 86 % of the 95 answers that were compared (72/95), with 39% (37/95) of the answers being more positive and 47 % (45/95) more negative.
Table 2 shows a breakdown of results of figure 1 into single functioning scales. The proportion of responses showing no change in relation to the standardized evaluation was low in all scales (11-27%), and in most scales, except for physical functioning and role functioning, the distribution between a more positive and a more negative rating was equal. Exceptions were the role functioning scale, where the majority (74%) of patients gave a more positive answer and the physical functioning scale, where the majority of patients (53%) gave a more negative answer.
Table 1:Coding of difference between QLQ and individual weighting.
| Weighting | Difference in % | Code |
|---|---|---|
| Much better | >75 to 100 | 4 |
| Significantly better | > 50 to ≥ 75 | 3 |
| Moderately better | > 25 to ≥ 50 | 2 |
| Slightly better | > 0 to ≥ 25 | 1 |
| Equal | 0 | 0 |
| Slightly worse | <0 to ≤-25 | -1 |
| Moderately worse | <-25 to ≤-50 | -2 |
| Significantly worse | <-50 to ≤-75 | -3 |
| Much worse | <-75 to≤-100 | -4 |
Table 2:Differences in functions as percentage of the patient population (coding method, table 1).
| Interpretation compared to QLQ* | Physical functioning | Role functioning | Emotional functioning | Social functioning | Cognitive functioning |
|---|---|---|---|---|---|
| Worse | 37 | 74 | 42 | 47 | 42 |
| Equal | 11 | 11 | 27 | 11 | 27 |
| Better | 53 | 27 | 42 | 42 | 42 |
| *in % to all answers in the functioning scale (n=95). | |||||
Table 3:(Patient characteristics, n=13).
| Age | Median 66 years (mean 65.08 ± 8.65) |
|---|---|
| Male/ female | 9/4 |
| Region of radiation | Bone 9/13 Cerebrum 4/13 |
| Disease | Solid tumors (lung, breast, prostate) 9/13 Plasmacytoma 2/13 Cerebral tumors 2/13 |
| Stage of disease | Symptomatic progressive disease after multiple therapies (operation, systemic drug therapies) |
Figure 1:Patients’ answers to the weighting question, differences in overall function (n=95).
Discussion
PROMS use validated questionnaires and are therefore standard tools in clinical studies in oncology. They might record different symptoms such as pain, tiredness, nausea, depression, anxiety, drowsiness, loss of appetite, diminishing of well-being, shortness of breath [12] or, other more complex aspects of QoL, such as physical, role, emotional, social and cognitive functioning (https://qol.eortc.org) that are evaluated by the EORTC QLQ C-30. The questionnaires that claim to capture the QoL as a whole, such as the EORTC QLQ C-30, have been developed to compare different therapies in treatment studies and not to depict health complaints or individual preferences for and expectations from a therapy.
There has been a change in patient characteristics over the last decade: the age of patients with newly diagnosed cancer is constantly increasing and, as a consequence, we have more patients with co-morbidities and co-medication, which increases the need of individualized therapies [13- 15]. More and more, advances in medicine have enabled such individually tailored therapies in oncology offering patients different medications with different side effects. In this context, the individual evaluation of complaints and expectations are warranted [16]. Life expectations, and consequently expectations from cancer therapies, are also different between younger and older people [15,17,18]. While the assessment of QoL as a whole is undoubtedly important in oncology, the individual weighting of the different aspects of QoL should also be recorded and taken into consideration with the aim of harmonizing the therapy goal with the patient’s expectations (“shared decision making”) this applies, in particular, in palliative oncology.
Only one questionnaire used in clinical studies, namely the Memorial Symptom Assessment Scale (MSAS), includes additional questions to find out whether the symptom with pronounced intensity is also important for the patient. The MSAS evaluates the overall prevalence, intensity, and frequency of about 33 different symptoms and, in addition, the distress associated with a particular symptom. The original questionnaire was developed in 1994 and a short version was released in 2020 [17,18]. Although the MSAS is quite suitable for evaluating therapy- or disease related symptoms, it does not cover all aspects of QoL as the EORTC QLQ C-30 does. However, such assessments as carried out by MSAS are necessary for shared decision making in which the life situation and expectations regarding QoL of the individual patient are taken into account.
In view of the fact that cancer patients included in our study were in a palliative radiation therapy scheme with the aim of improved QoL (table 3), we wanted to find out if the scores estimated by the original EORTC QLQ C-30 questionnaire would be different if the distress scale (“How would you currently rate your < functional scale> on a scale from 1 to 5”) were added to it. We found that the differences were striking, as has been shown in the results section. There were differences in 86 % (72/95) of the answers, 39% (37/95) of the answers being more positive and 47 % (45/95) were more negative when subjective weighting was added to items of the EORTC QoL questionnaire (figure 1). Interestingly, the discrepancies differed considerably between the various functioning scales (table 2). Whereas the proportion of responses showing no change to the standardized evaluation was low in all scales (11-27%), in most scales, except for physical functioning and role functioning, there was an equal distribution between a more positive and negative rating. The exceptions were the role functioning scale, where the majority (74%) of patients gave a more positive answer and the physical functioning scale, where the majority of patients (53%) gave a more negative answer (table 2).
We have no doubt that the EORTC QLQ C-30 is a powerful
instrument to evaluate the individual life quality at a given
time point. This has been shown in numerous studies since
its development in 1987 [19]. Nevertheless, whenever
improving or maintaining QoL is the primary goal in cancer
treatment, the addition of individual interpretation of the
different scales evaluated by the questionnaire is necessary,
be-cause QoL is a heterogeneous and highly individual
construct. The results of our study show that there is a
remarkable difference between the classical neutral and
impersonal interpretation of the questions and the balanced
interpretation that includes the weighting of symptoms by
the patients themselves. Interestingly, some of the weighting
was more positive, and some more negative as shown in
the results. Addition of a weighting question such as “how
do you experience your current situation regarding
We found that the differences were especially high in the interpretation of “role functioning” and, to a less extent, of “emotional functioning”. This might be due to the limitations of the study, these being the heterogeneous study group and the higher age of the patients, but the results are nevertheless noteworthy. Other dimensions such as “physical functioning” and “cognitive functioning” are easier to objectify and not so much subject to individual interpretation, which might be one possible explanation for smaller differences in these areas. Also, the limited number of possible answers (1 to 5) for each item in the weighting questions might have influenced the degree of deviation between the answers.
We and others think that one of the advantages of using weighting tools such as the question “does it bother you” should be used to improve therapy monitoring and to prevent therapy-associated problems. This is especially true when the responses are directly reported back to the treating doctor. Our small and limited study might serve as an inspiration to investigate the question in a larger patient group.
Declarations
Ethical approval and consent to participate
The project has been approved by the Ethical Committee of the University of Luebeck (file number 18-287). All patients have given a written consent to participate.
Availability of supporting data
All of questionnaires completed by the patients are stored in the original with the corresponding author
Conflict of interests
None All authors agreed to the publication.
Funding
Cancer Society of Schleswig-Holstein, Germany, grant 201905.
Authors’ contributions
GR: concept, methodology, validation, formal analysis, investigation, writing - original draft preparation, writing - review and editing. DR: resources, writing - review and editing, supervision. JD: resources, writing - review and editing, supervision. NvB: resources, writing - review and editing, project administration, funding acquisition. AL: concept, methodology, formal analysis, data curation management, writing - original draft preparation, writing - review and editing. FG: concept, methodology, validation, formal analysis, resources, data curation management, writing - original draft preparation, writing - review and editing, supervision, project administration, funding acquisition.
Acknowledgment
We thank KAIKU Health Ltd., Finland for providing their software platform KAIKU Health for cancer care and their active support in programming the questionnaires provided to patients on the tablet computers.
Deutschinoff G, Friedrich C, Thiem U, Voigtmann R, Pientka L (2005) Lebensqualität in der Onkologie. Der Onkologe 11: 164-172. [ Ref ]
Cull A, Sprangers M, Bjordal K, Aaronson N, West K, et al. (2002) EORTC quality of life group translation procedure: EORTC Brussels. [ Ref ]
Besson A, Deftereos I, Chan S, Faragher IG, Kinsella R, et al. (2019) Understanding patient-reported outcome measures in colorectal cancer. Future Oncology 15: 1135-1146. [ Ref ]
Koller M, Lorenz W (2002) Quality of Life: A Deconstruction for Clinicians. Journal of the Royal Society of Medicine 95: 481-488. [ Ref ]
Prinsen CA, Mokkink LB, Bouter LM, Alonso J, Patrick DL, et al. (2018) COSMIN guideline for systematic reviews of patient-reported outcome measures. Quality of Life Research 27: 1147-1157. [ Ref ]
Sprangers MA (2002) Quality-of-life assessment in oncology. Achievements and challenges. Acta Oncologica 41: 229-237. [ Ref ]
Luckett T, King M, Butow P, Friedlander M, Paris T (2010) Assessing health-related quality of life in gynecologic oncology: a systematic review of questionnaires and their ability to detect clinically important differences and change. International Journal of Gynecologic Cancer 20: 664-684. [ Ref ]
Moinpour CM, Feigl P, Metch B, Hayden KA, Meyskens Jr FL, et al. (1989) Quality of life end points in cancer clinical trials: review and recommendations. JNCI: Journal of the National Cancer Institute 81: 485-96. [ Ref ]
Field J, Holmes MM, Newell D (2019) PROMs data: can it be used to make decisions for individual patients? A narrative review. Patient related outcome measures 10: 233. [ Ref ]
Mackler ER, Weis T, Procailo KM, Marshall VD, Farris KB (2019) Utilizaton of patient reported outcomes measures (PROM) to characterize symptom burden and adherence associated with oral oncolytic therapy. American Society of Clinical Oncology 37: 210-210. [ Ref ]
Gilbert A, Selby P, Velikova G (2018) Monitoring of symptoms, toxicity and functioning using patient reported outcome measures. Problem Solving in Patient-Centred and Integrated Cancer Care. EBN Health, Witney, UK. [ Ref ]
Baba K, Fransson P, Lindh J (2007) Use of a modified ESAS in cancer patients: A pilot study of patient and staff experiences. International journal of palliative nursing 13: 610-616. [ Ref ]
Niess H, Kleespies A, Andrassy J, Pratschke S, Angele M, et al. (2013) Pancreatic cancer in the elderly: guidelines and individualized therapy. Der Chirurg; Zeitschrift fur alle Gebiete der operativen Medizen 84: 291- 295. [ Ref ]
Puts M, Papoutsis A, Springall E, Tourangeau A (2012) A systematic review of unmet needs of newly diagnosed older cancer patients undergoing active cancer treatment. Supportive Care in Cancer 20: 1377-1394. [ Ref ]
Salive ME (2013) Multimorbidity in older adults. Epidemiologic reviews 35: 75-83. [ Ref ]
Ely S (2009) Personalized medicine: individualized care of cancer patients. Translational Research 154: 303-308. [ Ref ]
van Weert JC, Bolle S, van Dulmen S, Jansen J (2013) Older cancer patients’ information and communication needs: what they want is what they get? Patient education and counseling 92: 388-397. [ Ref ]
Wan GJ, Counte MA, Cella DF (1997) The influence of personal expectations on cancer patients’ reports of health‐related quality of life. Psycho‐Oncology: Journal of the Psychological, Social and Behavioral Dimensions of Cancer 6: 1-11. [ Ref ]
Fayers P, Bottomley A, Group EQoL (2002) Quality of life research within the EORTC—the EORTC QLQ-C30. European Journal of Cancer 38: 125- 133. [ Ref ]