Journal Name: Journal of Health Science and Development
Article Type: Research
Received date: 05 April, 2022
Accepted date: 11 May, 2022
Published date: 2024-02-01
Citation: Liu X, Jiao G, Lian H, Liu L et al. (2022) Effects of Genistein Supplementation on the Characteristics of Human Sperm during Liquid Storage. J Health Sci Dev Vol: 5, Issue: 1 (22-30).
Copyright: © 2022 Liu X et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Cryopreservation, the most popular way to preserve human sperm, led to a significant decline in sperm motility. Here, we tried to introduce a new method to store sperm without freezing. Different concentrations of genistein were added to liquid preserved sperm. We investigated the effects of supplementation on sperm total antioxidative capacity (T-AOC), glutathione(GSH), methane dicarboxylic aldehyde (MDA), acrosomal enzyme activity and fertilization ability of sperm. The effects of liquid storage and cryopreservation on sperm parameters were also compared. IVF medium supplemented with genistein (20μmol L-1) maintained sperm motility for up to 11 days. The addition of genistein led to a decrease in reactive oxygen species (ROS) generation that demonstrated an effective improvement in sperm motility and decreased the MDA production and maintained the GSH content and enhanced the oxidative stress resistance ability of the sperm during liquid storage. The storage sperm were used for intracytoplasmic sperm injection(ICSI) into human oocytes and activated oocytes successfully. Sperm stored in liquid medium containing genistein was superior to sperm stored in liquid nitrogen in terms of antioxidant stress and fertilization ability. We confirmed that genistein could be used as an antioxidant for liquid storage of sperm. Sperm stored in IVF medium with genistein could avoid cryodamage, which may become an alternative option in assisted reproduction technology.
Keywords:
Antioxidant, Assisted reproductive technology, ROS, Sperm motility, Sperm preservation.
Abstract
Cryopreservation, the most popular way to preserve human sperm, led to a significant decline in sperm motility. Here, we tried to introduce a new method to store sperm without freezing. Different concentrations of genistein were added to liquid preserved sperm. We investigated the effects of supplementation on sperm total antioxidative capacity (T-AOC), glutathione(GSH), methane dicarboxylic aldehyde (MDA), acrosomal enzyme activity and fertilization ability of sperm. The effects of liquid storage and cryopreservation on sperm parameters were also compared. IVF medium supplemented with genistein (20μmol L-1) maintained sperm motility for up to 11 days. The addition of genistein led to a decrease in reactive oxygen species (ROS) generation that demonstrated an effective improvement in sperm motility and decreased the MDA production and maintained the GSH content and enhanced the oxidative stress resistance ability of the sperm during liquid storage. The storage sperm were used for intracytoplasmic sperm injection(ICSI) into human oocytes and activated oocytes successfully. Sperm stored in liquid medium containing genistein was superior to sperm stored in liquid nitrogen in terms of antioxidant stress and fertilization ability. We confirmed that genistein could be used as an antioxidant for liquid storage of sperm. Sperm stored in IVF medium with genistein could avoid cryodamage, which may become an alternative option in assisted reproduction technology.
Keywords:
Antioxidant, Assisted reproductive technology, ROS, Sperm motility, Sperm preservation.
Introduction
Cryopreservation of human sperm has been carried out in many fields, such as andrology laboratories and assisted reproduction centers. Human sperm cryopreservation shows a valuable therapeutic approach in management of male infertility [1]. However, there will lead to many adverse effects on sperm motility, viability and acrosome status when sperm are exposed to chemical and physical stresses during cryopreservation [2-6]. After cryopreservation, all these effects will reduce the fertilization ability of human sperm7. Alternative methods are required for the negative effects of sperm cryopreservation.
In animals, many methods of sperm preservation without freezing have been tried, such as preservation in sugars or salt and the evaporative drying [8-10]. In humans, sperm were attempted to be stored in a buffer containing egg yolk, Tris and TES without freezing up to 96 h [11-13]. However, sperm motility declined rapidly when sperm were stored in the buffer for 24 h [13]. It was reported that the frequency of structural changes increased when sperm chromosomes were evaluated after preservation [14,15]. Therefore, storage in this medium was considered mostly ineffective [16,17].
During sperm preservation, the occurrence of reactive oxygen species (ROS) would lead to oxidative stress [18]. The damage to sperm induced by ROS results in lipid peroxidation, which is caused by oxidative damage to sperm PUFAs (phospholipid-bound polyunsaturated fatty acids) [19,20]. There are various impacts of lipid peroxidation, such as decreased sperm motility, irreversible changes in sperm DNA and leakage of intracellular enzymes [21]. It also affects sperm penetration and prevents sperm-oocyte fusion [22]. Therefore, the generation of ROS needs to be reduced.
Genistein is an isoflavone that comes from soya or other legumes. Genistein inhibits protein tyrosine kinases and shows estrogen activity [23]. It modifies the capacitation and acrosome reaction process of mature sperm. Therefore, it affects a lot of functional parameters of mature sperm. In addition, the antioxidant capacity of genistein has been widely studied in vitro and also in vivo [24,25]. Furthermore, genistein can protect sperm DNA integrity through antioxidant activity [26].
In this study, we investigated the effects of the addition of genistein during liquid storage on sperm motility, T-AOC, GSH, MDA, acrosomal enzyme activity and fertilization ability of sperm. We compared the method of sperm storage in liquid with the traditional method of cryopreservation and found a new method to preserve sperm without freezing. Using this fundamental information, it would be possible to reduce the damage to sperm and improve the preservation process in ART.
Materials and Methods
Human sperm samples
The present study was approved by the institutional ethics committee review board of the Affiliated Yuhuangding Hospital of Qingdao University, Shandong, China. All men gave written informed consent prior to the start of the study. All semen samples were obtained from patients by masturbation who came for a semen analysis. These patients were abstinent from sexual activity for 2-7 days. Samples were collected into sterile containers and liquefied at room temperature. The samples were assessed for volume, pH, sperm concentration, percentage of sperm motility, percentage of normal morphology, and these analyses were according to the World Health Organization (WHO) guidelines [27].
Sperm preparation
Briefly, when the semen sample completed the liquefaction reaction, the entire semen was eased on the gradient medium consisting of 1.5 ml of 90% gradient medium and 1.5ml of 45% gradient medium. Afterwards, the column was centrifuged at 300×g for 20 min at room temperature. After the centrifugal procedure, the pellet sperm was resuspended in 2ml washing medium which was similar to medium used for storage. Then, the sperm was centrifuged again at 500×g for 10 min. At last, the supernatant part was separated and storage medium was added to the pellet to make a final concentration of 30-40×106 sperm/ml. It was then divided into a 1.5 ml tube with 0.5 ml sperm suspension /tube, sealed with mineral oil and stored for days at 24-26ºC.
Sperm storage
Sperm were preserved in these four different media, including IVF (10136, Vitrolife, Sweden), Human Tubal Fluid medium (MR-070-D, Sigma, USA), FM (K-SIFM-100, Cook, Australia) and PBS (Sers, China). Genistein was purchased from Sigma Chemical Co (G-6649, USA) and dissolved in DMSO (D2650, Sigma, USA) to yield a 10 mmol L−1 stock solution. The stock solution was divided into aliquots and frozen at -20ºC. The stock solution was diluted to the working solution with medium at the time of use. The cryopreservation method of sperm was as follows: the semen was centrifuged at 500×g for 10 min. Then, part of seminal plasma was removed and added equal volume cryoprotectant (ORIGIO, Denmark) and mixed well. Afterwards, the mixture was added to a 1.5 ml sperm cryopreservation tube and balanced at room temperature for 10min and fumigated above liquid nitrogen for 30 min. Finally, it was put into liquid nitrogen storage.
Sperm motility analysis
Sperm motility was analyzed by a computer-assisted sperm analyzer (CASA) system (IVOSII, Hamilton, USA). Then, a 5-μL drop aliquot of sperm suspension was placed on a pre-warmed microscope slide and overlaid with a 22 mm2 cover-slip. The slide was observed with a phase contrast microscope at 200 × magnification. Ten fields of view were evaluated and counted a minimum of 1000 sperm per sample. Sperm with a VAP < 10 μm s−1 were considered immotile.
Intracellular glutathione (GSH) measurement
Sperm samples were prepared as previously reported [28] with modifications. Briefly, 0.1 ml sperm suspension was placed in a centrifuge tube and centrifuged at 800×g for 15 min. After the supernatant was removed, the resulting sperm pellet was washed twice in Ca2+/Mg2+-free PBS by centrifugation at 400×g for 10 min. To release the intracellular content, the cells were broken by three cycles of rapid cooling in liquid nitrogen followed by thawing at 37ºC. Then, the resulting cell suspension was centrifuged at 7000×g for 10 min to remove membrane fragments, and the supernatant was stored at −80ºC until analyzed.
Intracellular GSH content was determined using a modified coupled optical test system [29]. In this system glutathione is oxidized by 5,5-dithiobis-(2-nitrobenzoic acid) (DTNB) and then reduced by glutathione reductase with NADPH as hydrogen donor. During the oxidation of glutathione by DTNB, 2-nitro-5 thiobenzoeic acid is formed, which can be detected photometrically by a change of absorption at 412nm. The content of reduced glutathione (GSH) is calculated according to a standard curve.
Total antioxidative capacity (T-AOC) measurement
T-AOC was measured with commercial kits using enzymatic methods (Jiancheng Technology, Nanjing, China). The determination of T-AOC followed the operating manual. Sperm were centrifuged at 800×g for 10 min at room temperature to obtain supernatant. The supernatant recovered was stored at -80°C before use. Briefly, 1000μl Reagent 1, 100μl sample, 2000μl Reagent 2 and 500μl Reagent 3 were used for the reaction. The control tube contained Reagent1, Reagent 2 and Reagent 3 without the addition of sample. The sample tube and the control tube took a 30 minute water bath at 37°C. Then 100μl Reagent 4 was added to the tubes and 100μl sample was added to the control tube. The last absorbance was taken at the end of the incubation period (10 min after the mixing). The absorption value was measured at 520nm. This assay relies on the ability of antioxidants in the sample to reduce Fe3+- TPTZ to Fe2+-TPTZ. The absorption value of the sample tube was corrected by the absorption value of the control tube.
Methane dicarboxylic aldehyde (MDA) measurement
MDA was measured with commercial kits using enzymatic methods (Jiancheng Technology, Nanjing, China). The determination of MDA followed the operating manual. Sperm were centrifuged at 800×g for 10 min at room temperature to obtain supernatant. The supernatant recovered was stored at −80°C before use. Briefly, 100μl Reagent1, 100μl sample, 3000μl Reagent 2 and 100μl Reagent 3 were used for the reaction. The control tube contained Reagent1, Reagent 2 and Reagent 3, and ethanol was added instead of the sample. The nozzle of the tubes was tightly covered with plastic film and a hole was punctured on top. The sample tube and control tube took a 40-minute water bath at 95°C and then centrifuged at 800×g for 10 min to obtain the supernatant. The absorption value of the supernatant was measured at 532nm. This determination depends on the reaction of MDA with TBA, which chemically binds to form another red substance. The red substance has a maximum absorption peak at 532nm. The absorption value of the sample tube was corrected by the absorption value of the control tube.
Acrosomal enzyme activity measurement
Acrosomal enzyme activity was measured with the quantitative assay kit (Huakang Medicine, Shenzhen, China). A modified Kennedy method was used to detect the acrosomal enzyme activity of sperm and follow the operating manual. The culture medium containing 7.5×106 sperm was centrifuged at 8000×g for 5 min to obtain the pellet at the bottom of the tube. After the supernatant was discarded, 100μl inhibitor was added to the tube and mixed well. 1000μl reaction buffer and 100μl stop buffer were added to the control tube, but only reaction buffer was added to the sample tube. The sample tube and the control tube took a 30-minute water bath at 37°C, then 100μl stop buffer was added to the sample tube. The tubes were centrifuged at 4000×g for 5 min to obtain supernatant. The absorption value of the supernatant was measured at 410nm. This assay relies on the reaction of arginine amidase with nitroaniline, which chemically binds to form another colored substance. The absorption value of the sample tube was corrected by the absorption value of the control tube. The value was calculated as follows: (sample OD-control OD) ×106/ (247.5×7.5).
Intracytoplasmic sperm injection
The MII-stage oocytes used for ICSI were collected from MI-stage oocytes discarded by ICSI patients, which cultured in IVF medium for 20-24h in vitro. Sperm with the fastest movement and normal morphology were selected for injection. After injection, oocytes were transferred to GI medium (10128, Vitrolife, Sweden) and cultured at 37.0°C. The fertilization rate of oocytes was scored after microinjection for 17-20h. The oocytes with two welldeveloped pronuclei and extruded second polar body were considered activated. On day 3, a quality score was given for cultured embryos, and those with 7-9 uniformly sized cleavage balls and a fragmentation rate of less than 20% were considered high-quality embryos. Embryos were transferred to GII medium (10132, Vitrolife, Sweden) for further development on the afternoon of day 3. On day 5 and day 6, only blastocysts with dilated blastocyst cavity, clearly visible inner cell masses and compact trophoblast cells were considered as available blastocysts.
Statistical Analysis
There were at least three replicates for each treatment. Percentage data were arc sine transformed and analyzed with ANOVA when each measure contained more than two groups or with an independent t-test when each measure had only two groups; a Duncan multiple comparison test was used to locate differences. The software used was Statistics Package for Social Science (SPSS 11.5; SPSS Inc., Chicago, IL, USA). Data were expressed as mean ± S.E.M. and P<0.05 was considered significant.
Results
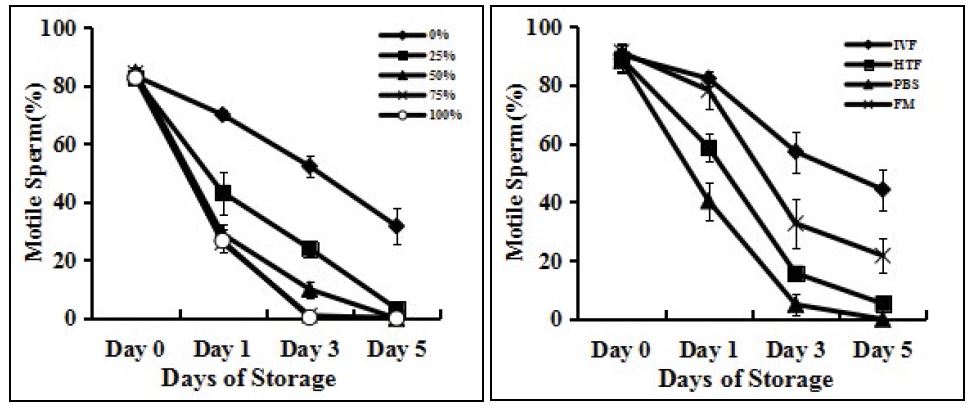
Effect of seminal plasma on sperm motility
Sperm motility declined rapidly when stored as an unwashed semen (Figure 1A) and on day 5, average sperm motility was less than 1%. When the ratio of seminal plasma was more than 50%, sperm motility decreased quickly compared with sperm without seminal plasma (p<0.05). Unwashed semen storage caused rapid deterioration of sperm. It was not suitable for sperm storage.
Effect of different media on sperm motility
The motility of sperm stored in HTF and PBS medium decreased rapidly on the first day compared to sperm stored in IVF and FM medium. On day 3, the motility of sperm stored in FM medium began to decrease quickly, about a 50% reduction. The motility of sperm preserved with IVF medium was maintained longer than that of sperm stored in FM medium (Fig.1B). The average motility of sperm stored in IVF and FM medium for 5 days was 44.3±7.0% and 21.7±5.9% (p<0.05) respectively, about two times. Sperm motility in IVF medium was much better maintained than in FM medium. Thus, in the following experiments, sperm were always stored in IVF medium.
Figure 1:Effects of different media and proportions of seminal plasma on the motile sperm percentages. (A) Sperm were stored in IVF medium with different proportions of seminal plasma at 24-26°C for 5 days. Sperm motility was assessed every other day. (B) Sperm were stored in IVF, HTF, PBS and FM medium, free of seminal plasma at 24-26°C for 5 days. Sperm motility was assessed every other day. Each data point represents the mean ± SEM.
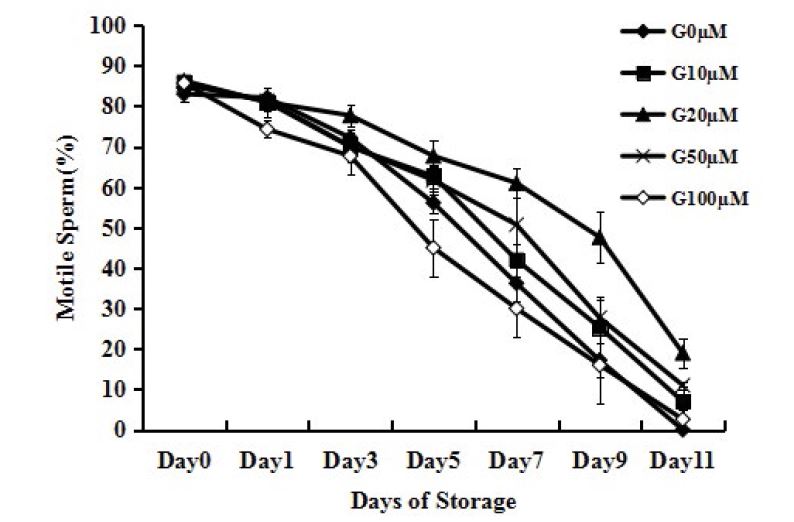
Effect of different concentrations genistein on sperm motility
Addition of genistein (20μM, 50μM) to IVF medium increased the percentage of motile sperm (Figure 2, P<0.05). However, sperm treated with genistein (100μM) showed lower motility than those without genistein (Figure 2, P<0.05). No difference was found between adding genistein (10μM) group and no adding genistein group. The genistein (20μM) group maintained sperm motility better than the genistein (50μM) group. The difference between the two groups was statistically significant after 7 days of storage (Figure 2, P<0.05). On day 7, the average motility of the two groups was 61.0±3.6% and 42.0±4.0%, respectively.
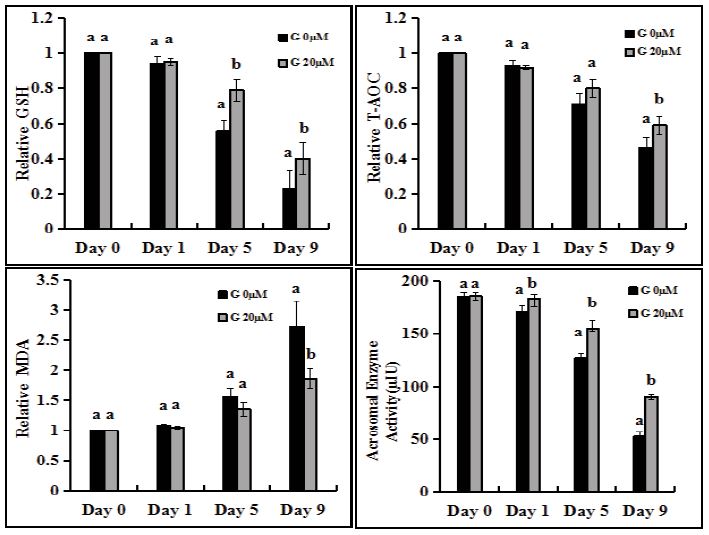
Effect of genistein and cryopreservation on sperm ability to resist oxidative stress
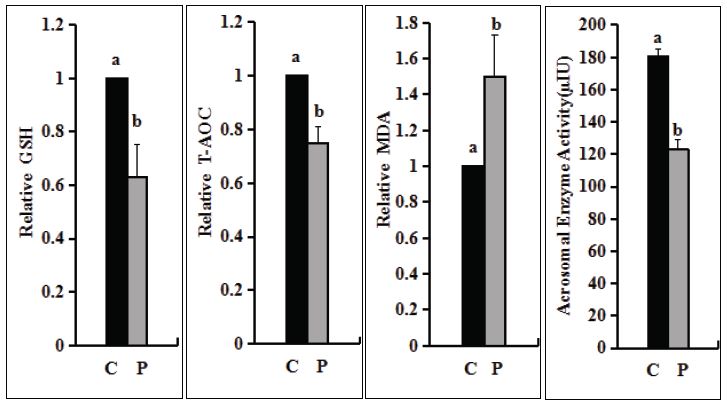
The T-AOC and GSH decreased gradually, but the addition of genistein could delay the decrease. From day 5, genistein supplementation could maintain GSH, which was significantly higher than that of non-additive group (Figure 3A, P<0.05). The two groups were also significantly different on day 9 (Figure 3A, Figure 3B, P<0.05). The MDA increased gradually, but the addition of genistein could delay the increase. There was significant difference between the two groups on day 9 (Figure 3C, P<0.05). The GSH and T-AOC were also significantly reduced and the MDA was significantly increased after the preservation of sperm in liquid nitrogen (Figure 4A-C, P<0.05), indicating that the ability of sperm to resist oxidative stress was significantly reduced. The results showed that the antioxidant stress ability of sperm in liquid storage for less than 5 days should be higher than that of sperm after liquid nitrogen storage.
Effect of genistein and cryopreservation on acrosomal enzyme activity of sperm
From day 5, the activity of sperm acrosomal enzyme was maintained by adding genistein, which was significantly higher than that of the non-additive group (Figure 3D, P<0.05). On day 9, the acrosomal enzyme activity of sperm in the two groups was 90.7±2.3μIU and 52.3±4.3μIU (p<0.05) respectively, about two times. The acrosomal enzyme activity decreased from 180.3±4.7μIU to 123.2±5.7μIU after sperm were stored in liquid nitrogen (Figure 4D, P<0.05). The results showed that adding genistein may be an optimal method to maintain the acrosomal enzyme activity of sperm.
Effect of genistein and cryopreservation on sperm fertilization ability
Sperm were stored in IVF medium with addition of genistein (20μM) for different days and then used for ICSI into human oocytes to assess their ability to participate in assisted fertilization and embryo development (Table 1). Fresh sperm were injected as controls. Fertilization rates of oocytes were similar except the group that sperm stored in IVF medium without addition of genistein on day 9 when the motility of sperm was very low. There was no difference in embryo cleavage rate between all the groups. High quality embryo and blastocyst formation of sperm without addition of genistein on day 9 was significantly lower than that of other groups. The results showed that the sperm fertilization ability of genistein added on day 5 was similar to that of fresh sperm. After the sperm were stored in liquid nitrogen, the fertilization ability was similar to that of the sperm without adding genistein on day 5. Overall, the results demonstrated that sperm preserved in IVF medium with genistein for several days were functional in assisted fertilization.
Discussion
In this study, we showed that preservation of human sperm in IVF medium with genistein could maintain sperm motility for several days. Compared with the traditional cryopreservation method, this method can maintain sperm motility and antioxidant stress ability in a short time. It may be an alternative method for sperm preservation.
Figure 2:Effects of different concentrations of genistein supplementation on the motile sperm percentages. Sperm were stored in IVF medium with different concentrations of genistein at 24-26°C for 11 days. Each data point represents the mean ± SEM.
Figure 3:Effects of genistein supplementation on GSH, T-AOC, MDA, acrosomal enzyme activity of sperm. (A) Measurement of relative GSH content. (B) Measurement of relative T-AOC content. (C) Measurement of relative MDA content. The data(A-C) of day 0 was set to one and the data of other days were compared with the data of day 0. (D) Measurement of acrosomal enzyme activity. Each graph bar represents the mean ± SEM. Different letters within an assessment significantly different at P <0 .05.
Figure 4:Effects of cryopreservation on T-AOC, GSH, MDA, acrosomal enzyme activity of sperm. (A) Measurement of relative GSH content. (B) Measurement of relative T-AOC content. (C) Measurement of relative MDA content. The data(A-C) of day 0 was set to one and the data of other days were compared with the data of day 0. (D) Measurement of acrosomal enzyme activity. “C” means sperm without liquid nitrogen preservation. “P” means that sperm had been preserved in liquid nitrogen. Each graph bar represents the mean ± SEM. Different letters within an assessment significantly different at P <0 .05.
Table 1:Effects of genistein supplementation and cryopreservation on sperm fertilization ability and embryo development.
| Treatments | No.of oocytes injected | No.of oocytes fertilized(%)b | No.of embryos cleavage (%)c | No.of high quality embryos(%)d | No.of available blastocyst formation(%)e |
|---|---|---|---|---|---|
| G0μM | |||||
| Day 0 | 25 | 19(76) | 17(95) | 9(53) | 5(29) |
| Day 1 | 27 | 22(81) | 20(91) | 9(45) | 5(25) |
| Day5 | 23 | 16(70) | 14(88) | 5(36) | 2(14) |
| Day 9 | 20 | 11(55) | 9(82) | 1(11) | 0(0) |
| G20μM | |||||
| Day 1 | 28 | 24(86) | 22(92) | 12(55) | 7(32) |
| Day5 | 25 | 20(80) | 18(90) | 9(50) | 5(28) |
| Day 9 | 21 | 14(67) | 12(86) | 3(25) | 1(8) |
| Preservation | 24 | 17(71) | 15(88) | 6(40) | 2(13) |
| aICSI was done with motile sperm only. bPercentage was calculated from oocytes injected. cPercentage was calculated from oocytes fertilized. dPercentage was calculated from embryos cleavage. ePercentage was calculated from embryos cleavage. | |||||
At present, cryopreservation of sperm is the only way to store human sperm, but this common method leads to a significant decline in sperm motility [30]. Cryopreservation leads to sperm DNA damage and may also bring hidden effects such as changed styles of intracellular enzyme activities and disturbed plasma membranes undetectable by supravital staining [31,32]. It has been demonstrated that sperm DNA damage leads to reduced reproductive outcomes such as adverse effects on fertilization rate and preimplantation development. It also causes pregnancy loss and morbidity [33]. Hence, it is important to keep sperm DNA integrity for the right conveyance of chromatin materials to the next generation. For successful assisted reproduction, it is necessary to develop a better way to maintain sperm DNA than cryopreservation.
Sperm stored without washing may be the simplest method of preservation without freezing, but sperm motility lost rapidly on the first day. Seminal plasma may contain something detrimental to sperm [34]. Seminal plasma can potentially protect sperm from DNA damage for containing a lot of antioxidant enzymes, but it also reduces sperm viability significantly for containing bacteria and leukocytes. However, seminal plasma also contains nucleases, which are detrimental to sperm with damaged membrane because it can enter and digest sperm DNA [35,36].
In our study, sperm preserved in HTF and PBS media suffered rapid quality loss. After three days of storage, sperm motility decreased rapidly in most media except IVF medium. When the four different media were compared, IVF medium was the best one for sperm storage that could maintain sperm quality well. It was reported that sperm motility declined to below 10% within a few days of being stored in HTF [37]. A lot of research has been carried out on the origin of DNA damage [38]. Recently, it was proved that sperm motility could be maintained long enough to participate in fertilization in vivo due to the presence of pro-survival factors. These pro-survival factors can prevent sperm from entering an apoptotic state [39]. These pro-survival factors, which may be present in IVF media rather than other media, can prevent sperm from entering the apoptotic pathway, or at least slow it down. Consequently, if the simple medium doesn’t contain pro-survival factors, it may facilitate sperm entry into the apoptotic pathway.
Reactive oxygen species directly caused sperm DNA damage [40]. The results showed that ROS damaged sperm nucleus and mitochondria, which were compatible with keeping sperm motility and fertilization ability [41]. The metabolic processes of sperm and exogenous chemicals produced ROS, which damaged sperm biological system. During cryopreservation, it has been proven that lipid peroxidation of sperm membrane increased probably because of ROS release [42]. Sperm were particularly vulnerable to ROS-induced damage because they contained large amounts of PUFA content. Thus, it was necessary to add antioxidants to reduce the effect of ROS on sperm [43].
Changes in redox balance between defense mechanisms and ROS generation were associated with sperm capacitation process [44]. Sperm motility and capacitation were related to protein phosphorylation. The inhibition of tyrosine kinase caused changes of sperm motility parameters [45]. Genistein is an inhibitor of tyrosine kinase that affects sperm motility in a dose-dependent manner. Low concentrations of genistein did not interfere with sperm motility in mice and human, but high doses had a negative effect on sperm motility, and then showed that sperm motility decreases with the addition of 400μmol/L genistein [45-47]. Here, we showed that sperm motility had a slight increase when 20μmol/L genistein was added to the liquid storage medium. The addition of genistein reduced the ROS generated during the liquid storage of sperm and these data indicated that genistein used at this concentration (20μmol/L) could offer antioxidant properties to sperm. We speculated that it was associated with the inhibition of tyrosine kinase and the process of capacitation [45].
In previous experiments, reduced glutathione was added to the medium to increase the percentage of viable sperm. During liquid storage, the increased oxidative stress was associated with the negative effects of ROS. The decrease of GRD activity and SOD activity led to the decrease of GSH content and the increase of superoxide anion formation. GSH oxidation also increased because of hydrogen peroxide. Increased ROS production and decreased intracellular GSH during fluid storage lead to decreased sperm motility. The addition of exogenous genistein during liquid storage would prevent ROS-induced damage and maintain sperm function. The results showed that low-dose genistein treatment could increase the serum testosterone level in mice and regulate expression of spermatogenesis related genes. Expressions of ESR2, CYP19A1, SOX9 and BRD7 in mouse testis increased after genistein treatment [48]. Estrogen receptors (ESR2) mediated the effects of estrogen [49]. CYP19A1 genes played an important role in estrogen biosynthesis [50,51]. SOX9 was an indispensable regulator of germ cell survival and proliferation [52]. Genistein interacted with estrogen receptors and had important estrogen effects [53-55]. Hence, we speculated that genistein may play its role on sperm by regulating the expression of certain genes.
The method of storing sperm in liquid can be applied to the following types: Firstly, sperm with poor freezing resistance have normal motility before freezing, but the sperm quality of the patients decreases significantly after cryopreservation. There are pathological changes in the structure and function of sperm in some or many aspects. These congenital defects could make sperm vulnerable to freezing damage, resulting in poor freezing performance. Secondly, low quality sperm refers to a group of pathological semen specimens with abnormal semen routine parameters or low sperm basic functions, such as rare, weak and abnormal sperm. Low quality sperm have worse antifreeze performance and changes in sperm function may have a greater impact. Thirdly, patients who have difficulty in masturbation do not need long-term sperm storage, and liquid storage may be a more appropriate method.
In summary, we have shown that the isoflavone genistein has antioxidant properties when added to the liquid storage medium of sperm. Genistein caused a decrease in ROS production and an increase in sperm motility. This shortterm storage may be the best way to store human sperm without freezing. This approach could provide high-quality preserved sperm for subsequent assisted fertilization and may become a fundamental method for ART.
Contribution of Authors
Guangzhong Jiao and Xiaoyan Liu: study design, conception of the research idea and drafting the manuscript; Huayu Lian and Ling Liu:performed the experiments and data collection; Lili Chen and Luping Zhang:data analysis. All authors mentioned above have thoroughly read and approved the manuscript.
Acknowledgements
This study was supported by the Shandong Natural Science Foundation (ZR2016HL08) and the Yuhuangding Hospital Foundation (201518).
Disclosure Statement
No potential conflflict of interest was reported by the author(s).
Oehninger S, Duru NK, Srisombut C, Morshedi M (2000) Assessment of sperm cryodamage and strategies to improve outcome. Mol Cell Endocrinol 169: 3-10. [ Ref ]
O’Connell M, McClure N, Lewis SE (2002) The effects of cryopreservation on sperm morphology, motility and mitochondrial function. Hum Reprod 17: 704-709. [ Ref ]
Schiller J, Arnhold J, Glander HJ, Arnold K (2000) Lipid analysis of human spermatozoa and seminal plasma by MALDITOF mass spectrometry and NMR spectroscopy–effects of freezing and thawing. Chem Phys Lipids 106:145-156. [ Ref ]
Critser JK, Arneson BW, Aaker DV, Huse-Benda AR, Ball GD (1987) Cryopreservation of human spermatozoa. II. Postthaw chronology of motility and of zona-free hamster ova penetration. Fertil Steril 47: 980- 984. [ Ref ]
Alvarez JG, Storey BT (1993) Evidence that membrane stress contributes more than lipid peroxidation to sublethal cryodamage in cryopreserved human sperm: glycerol and other polyols as sole cryoprotectant. J Androl 14: 199-209. [ Ref ]
McLaughlin EA, Ford WC, Hull MG (1992) The contribution of the toxicity of a glycerol-egg yolk-citrate cryopreservative to the decline in human sperm motility during cryopreservation. J Reprod Fertil 95: 749-754. [ Ref ]
Mack SR, Zaneveld LJ (1987) Acrosomal enzymes and ultrastructure of unfrozen and cryotreated human spermatozoa. Gamete Res 18: 375- 383. [ Ref ]
Ono T, Mizutani E, Li C, Wakayama T (2010) Preservation of sperm within the mouse cauda epididymidis in salt or sugars at room temperature. Zygote 18:245-256. [ Ref ]
Bhowmick S, Zhu L, McGinnis L, Lawitts J, Nath BD, et al. (2003) Desiccation tolerance of spermatozoa dried at ambient temperature: production of fetal mice. Biol Reprod 68: 1779-1786. [ Ref ]
McGinnis LK, Zhu L, Lawitts JA, Bhowmick S, Toner M, et al. (2005) Mouse sperm desiccated and stored in trehalose medium without freezing. Biol Reprod 73: 627-633. [ Ref ]
Jaskey DG, Cohen MR (1981) Twenty-four to ninety-six-hour storage of human spermatozoa in test-yolk buffer. Fertil Steril 35: 205-208. [ Ref ]
Bolanos JR, Overstreet JW, Katz DF (1983) Human sperm penetration of zonafree hamster eggs after storage of the semen for 48 hours at 2 degrees C to 5 degrees C. Fertil Steril 39: 536-541. [ Ref ]
Kesseru E, Carrere C (1984) Duration of vitality and migrating ability of human spermatozoa cryopreserved at þ4 degrees C. Andrologia 16: 429-433. [ Ref ]
Martin RH, Templado C, Ko E, Rademaker A (1990) Effect of culture conditions and media on the frequency of chromosomal abnormalities in human sperm chromosome complements. Mol Reprod Dev 26: 101- 104. [ Ref ]
Munne S, Estop AM (1993) Chromosome analysis of human spermatozoa stored in vitro. Hum Reprod 8: 581- 586. [ Ref ]
Muratori M, Maggi M, Spinelli S, Filimberti E, Forti G, et al. (2003) Spontaneous DNA fragmentation in swim-up selected human spermatozoa during long term incubation. J Androl 24: 253-262. [ Ref ]
Schuffner A, Morshedi M, Vaamonde D, Duran EH, Oehninger S (2002) Effect of different incubation conditions on phosphatidylserine externalization and motion parameters of purified fractions of highly motile human spermatozoa. J Androl 23: 194-201. [ Ref ]
Chatterjee S, Gagnon C (2001) Production of reactive oxygenspecies by spermatozoa undergoing cooling, freezing, and thawing. Mol Reprod Dev 59: 451-458. [ Ref ]
Aitken RJ (1989) The role of free oxygen radicals and sperm function. Int J Androl 12: 95-97. [ Ref ]
Alvarez JG, Storey BT (1995) Differential incorporation of fatty acids into and peroxidative loss of fatty acids from phospholipids of human spermatozoa. Mol Reprod Dev 42: 334-346. [ Ref ]
White IG (1993) Lipids and calcium uptake of sperm in relation to cold shock and preservation: a review. Reprod Fertil Dev 5: 639-658. [ Ref ]
Aitken RJ (1995) Free radicals, lipid peroxidation and sperm function. Reprod Fertil Dev 7: 659-668. [ Ref ]
Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, et al. (1987) Genistein, a specifi inhibitor of tyrosine-specifi protein kinases. J Biol Chem 262: 5592-5595. [ Ref ]
Sierens J, Hartley JA, Campbell MJ, Leathem AJ, Woodside JV (2001) Effect of phytoestrogen and antioxidant supplementation on oxidative DNA damage assessed using the comet assay. Mutat Res 485: 169-176. [ Ref ]
Mitchell JH, Cawood E, Kinniburgh D, Provan A, Collins AR, et al. (2001) Effect of a phytoestrogen food supplement on reproductive health in normal males. Clin Sci (Lond) 100: 613-618. [ Ref ]
Sierens J, Hartley JA, Campbell MJ, Leathem AJ, Woodside JV (2002) In vitro isoflvone supplementation reduces hydrogen peroxide-induced DNA damage in sperm. Teratog Carcinog Mutagen 22: 227-234. [ Ref ]
World Health Organization (1999) WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus interaction. Cambridge University Press, Cambridge. [ Ref ]
Stradaioli G, Noro T, Sylla L, Monaci M (2007) Decrease in glutathione (GSH) content in bovine sperm after cryopreservation: comparison between two extenders. Theriogenology 67: 1249-1255. [ Ref ]
Gadea J, Selles E, Marco MA, Coy P, Matas C, et al. (2004) Decrease in glutathione content in boar sperm after cryopreservation. Effect of the addition of reduced glutathione to the freezing and thawing extenders. Theriogenology 62: 690-701. [ Ref ]
Zribi N, Feki Chakroun N, El Euch H, Gargouri J, Bahloul A, et al. (2010) Effects of cryopreservation on human sperm deoxyribonucleic acid integrity. Fertil Steril 93: 159-166. [ Ref ]
Toro E, Fernandez S, Colomar A, Casanovas A, Alvarez JG, et al. (2009) Processing of semen can result in increased sperm DNA fragmentation. Fertil Steril 92: 2109-2112. [ Ref ]
Glander HJ, Schaller J (2000) Hidden effects of cryopreservation on quality of human spermatozoa. Cell Tissue Bank 1: 133-142. [ Ref ]
Simon L, Brunborg G, Stevenson M, Lutton D, McManus J, et al. (2010) Clinical significance of sperm DNA damage in assisted reproduction outcome. Hum Reprod 25: 1594-1608. [ Ref ]
Riel JM, Huang TT, Ward MA (2007) Freezing-free preservation of human spermatozoa—a pilot study. Arch Androl 53: 275-284. [ Ref ]
Lewis SE, Sterling ES, Young IS, Thompson W (1997) Comparison of individual antioxidants of sperm and seminal plasma in fertile and infertile men. Fertil Steril 67: 142-147. [ Ref ]
Mann T, Lutwak-Mann C (1981) Male Reproductive Function and Semen. Springer-Verlag, Berlin. [ Ref ]
Quan S, Zhou HK, Shuji Y, Hisayo N, Toshihiro A (2002) Fertilizing capacity of human sperm preserved in cold electrolyte-free solution. Di Yi Jun Yi Da Xue Xue Bao 22: 928-930. [ Ref ]
Sakkas D, Alvarez JG (2010) Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril 93: 1027-1036. [ Ref ]
Aitken RJ, Koppers AJ (2011) Apoptosis and DNA damage in human spermatozoa. Asian J Androl 13:36-42. [ Ref ]
Aitken RJ, De Iuliis GN, McLachlan RI (2009) Biological and clinicalsignificance of DNA damage in the male germ line. Int J Androl 32: 46-56. [ Ref ]
Aitken RJ, Gordon E, Harkiss D, Twigg JP, Milne P, et al. (1998) Relative impact of oxidative stress on the functional competence andgenomic integrity of human spermatozoa. Biol Reprod 59: 1037-1046. [ Ref ]
Alvarez JG, Storey BT (1992) Evidence for increased lipid peroxidative damage and loss of superoxide dismutase activity as a mode of sublethal cryodamage to human sperm during cryopreservation. J Androl 13: 232-241. [ Ref ]
Gomez E, Buckingham DW, Brindle J, Lanzafame F, Irvine DS, et al. (1996) Development of an image analysis system to monitor the retention of residual cytoplasm by human spermatozoa: correlation with biochemical markers of the cytoplasmic space, oxidative stress, and sperm function. J Androl 17: 276-287. [ Ref ]
Griveau JF, Le Lannou D (1997) Reactive oxygen species and human spermatozoa: physiology and pathology. Int J Androl 20: 61-69. [ Ref ]
Bajpai M, Asin S, Doncel GF (2003) Effect of tyrosine kinase inhibitors on tyrosine phosphorylation and motility parameters in human sperm. Arch Androl 49: 229-246. [ Ref ]
Kumi-Diaka J, Townsend J (2001) Effects of genistein isoflvone(4′,5 ′,7-trihydroxyisoflavone) and dexamethasone on functional characteristics of spermatozoa. J Med Food 4: 39-47. [ Ref ]
Bajpai M, Doncel GF (2003) Involvement of tyrosine kinase and cAMP-dependent kinase cross-talk in the regulation of human sperm motility. Reproduction 126: 183-195. [ Ref ]
Shi Z, Lv Z, Hu C, Zhang Q, Wang Z, et al. (2020) Oral Exposure to Genistein during Conception and Lactation Period Affects the Testicular Development of Male Offspring Mice. Animals (Basel) 10: 377. [ Ref ]
Rago V, Romeo F, Giordano F, Malivindi R, Pezzi V, et al. (2018) Expression of oestrogen receptors (GPER, ESR 1, ESR 2) in human ductuli efferentes and proximal epididymis. Andrology 6: 192-198. [ Ref ]
Coban N, Gulec C, Ozsait-Selcuk B, Erginel-Unaltuna N (2017) CYP19A1, MIF and ABCA1 genes are targets of the RORα in monocyte and endothelial cells. Cell Biol Int 1: 163-176. [ Ref ]
Nakamoto M, Shibata Y, Ohno K, Usami T, Kamei Y, et al. (2018) Ovarian aromatase loss-of-function mutant medaka undergo ovary degeneration and partial female-to-male sex reversal after puberty. Mol Cell Endocrinol 460: 104-122. [ Ref ]
Wang H, Zhao R, Guo C, Jiang S, Yang J, et al. (2016) Knockout of BRD7 results in impaired spermatogenesis and male infertility. Sci Rep 6: 1-13. [ Ref ]
Swart AC, Johannes ID, Sathyapalan T, Atkin SL (2019) The Effect of Soy Isoflavones on Steroid Metabolism. Front Endocrinol 10: 229. [ Ref ]
Mukund V, Mukund D, Sharma V, Mannarapu M, Alam A (2017) Genistein: Its role in metabolic diseases and cancer. Crit Rev Oncol Hematol 119: 13-22. [ Ref ]
De Gregorio C, Marini H, Alibrandi A, Di Benedetto A, Bitto A, et al. (2017) Genistein supplementation and cardiac function in postmenopausal women with metabolic syndrome: Results from a pilot strain-echo study. Nutrients 9: 584. [ Ref ]