Journal Name: Journal of Health Science and Development
Article Type: Research
Received date: 19 April, 2024
Accepted date: 09 May, 2024
Published date: 16 May, 2024
Citation: Arora S, Grandhi B, Vakhariya S (2024) Exploring Constraints in the Acceptance of Glucagon-Like Peptide Antagonists for the Management of Diabetes and Obesity in India: A Comprehensive Study and Innovative Strategy Design. J Health Sci Dev Vol: 7, Issue: 1 (28-34).
Copyright: © 2024 Arora S et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
This study sets out to identify and elucidate the primary constraints that hinder the acceptance of Glucagon-like Peptide Antagonists in the effective management of diabetes and obesity. Additionally, the research aims to pioneer an innovative strategy to overcome these identified constraints successfully. A notable challenge in this context is the pricing of innovative drugs, specifically those designed to address Type 2 Diabetes Mellitus (T2DM) and its prevalent comorbidity, obesity. Despite the promising emergence of medications such as GLP1 analogs in the Indian market, their prohibitive costs and limited awareness about the molecule, attributed to substantial research and development investments, pose a significant barrier.
The pricing strategy, crafted to maximize profits during exclusivity periods, imposes restrictions on market share and accessibility. This is particularly noteworthy in India, where a substantial proportion of patients falls into the self-pay category, often lacking extensive financial planning for healthcare. The study will also delve into multifaceted aspects such as financing schemes, market access strategies, market liberalization, internet trading, and the impact of biosimilars on pricing, providing a comprehensive understanding of the intricate landscape.
To address existing gaps in discussions related to discounts, rebates, profits, and price transparency, this research aims to introduce the New Product Pricing Model (NPPM). Furthermore, it advocates for increased dissemination of long-term data on GLP1 by multinational companies to healthcare professionals (HCPs), emphasizing the importance of designing an efficient brand-building strategy. This comprehensive approach is expected to not only enhance the accessibility of anti-diabetic and anti- obesity products in India but also foster a greater understanding and appreciation of GLP1 among healthcare professionals.
Keywords
Diabetes, Obesity, Self-Pay, GLP1 Analogues (GLP1 Analogs), New Product Pricing Model, Market Access Strategies, Market Liberalization
Abstract
This study sets out to identify and elucidate the primary constraints that hinder the acceptance of Glucagon-like Peptide Antagonists in the effective management of diabetes and obesity. Additionally, the research aims to pioneer an innovative strategy to overcome these identified constraints successfully. A notable challenge in this context is the pricing of innovative drugs, specifically those designed to address Type 2 Diabetes Mellitus (T2DM) and its prevalent comorbidity, obesity. Despite the promising emergence of medications such as GLP1 analogs in the Indian market, their prohibitive costs and limited awareness about the molecule, attributed to substantial research and development investments, pose a significant barrier.
The pricing strategy, crafted to maximize profits during exclusivity periods, imposes restrictions on market share and accessibility. This is particularly noteworthy in India, where a substantial proportion of patients falls into the self-pay category, often lacking extensive financial planning for healthcare. The study will also delve into multifaceted aspects such as financing schemes, market access strategies, market liberalization, internet trading, and the impact of biosimilars on pricing, providing a comprehensive understanding of the intricate landscape.
To address existing gaps in discussions related to discounts, rebates, profits, and price transparency, this research aims to introduce the New Product Pricing Model (NPPM). Furthermore, it advocates for increased dissemination of long-term data on GLP1 by multinational companies to healthcare professionals (HCPs), emphasizing the importance of designing an efficient brand-building strategy. This comprehensive approach is expected to not only enhance the accessibility of anti-diabetic and anti- obesity products in India but also foster a greater understanding and appreciation of GLP1 among healthcare professionals.
Keywords
Diabetes, Obesity, Self-Pay, GLP1 Analogues (GLP1 Analogs), New Product Pricing Model, Market Access Strategies, Market Liberalization
Background
Diabetes mellitus (DM) is a complex, progressive condition that heightens the susceptibility of patients to various health complications. According to the most recent estimates from the International Diabetes Federation (IDF), approximately 8.3% of adults globally (382 million) are affected by diabetes, with India reporting a prevalence of 9.09% (65 million). Notably, a baseline study involving 20,554 Indian subjects with type 2 DM in the A1chieve study revealed a high incidence of both macrovascular and microvascular complications, primarily attributed to inadequate glycemic control (mean HbA1c = 9.2 ± 1.4).
Key studies such as UKPDS (66,000 patient years of exposure [PYE] follow up), DCCT (an average of 23.5 years of follow up), and STENO-2 (13.3 years follow up) have underscored the significance of intensive glycemic therapy. They indicate a substantial reduction in the risk of macrovascular and microvascular complications, a sustained legacy effect despite early normalization of HbA1c levels, and an overall reduction in the risk of diabetes-related endpoints. Maintaining adequate glycemic control is crucial, yet it is challenging over the course of type 2 diabetes progression, necessitating diverse treatment options.
The landscape of type 2 diabetes management is evolving with an expanding array of pharmacological agents. A joint committee by the American Diabetic Association (ADA) and the European Association for the Study of Diabetes (EASD) highlighted the need to evaluate available therapies based on factors like efficacy, hypoglycemia, weight, major side effects, and cost [1]. This framework assists clinicians and patients in devising a tailored plan to meet treatment goals.
Weight gain is often associated with type 2 DM, posing a barrier to treatment intensification and increasing cardiovascular risk. The unmet needs in managing type 2 diabetes include the progressive decline in beta-cell function, dysregulated glucagon release, reduced incretin effect, and weight gain, which are not adequately addressed by existing therapies. GLP-1 analogues have emerged as promising solutions, addressing these issues by inducing glucosedependent insulin release, inhibiting glucagon release, delaying gastric emptying, enhancing satiety, reducing energy intake, and improving insulin sensitivity [2].
Consequently, GLP-1 analogues offer a comprehensive and appealing treatment option for diabetes management.
Internationally renowned weight loss medications include Novo Nordisk’s Wegovy/Ozempic (semaglutide) and Saxenda (liraglutide), both of which target the GLP1 hormone, effectively inducing a sense of fullness and diminishing food intake. Eli Lilly’s Mounjaro (tirzepatide) takes a distinctive approach by combining the GLP-1 and glucose- dependent insulinotropic peptide (GIP) hormones produced in the gut to facilitate weight loss.
In the Indian context, the spectrum of weight loss options is somewhat limited, encompassing medications such as orlistat, metformin, and liraglutide (Victoza). Metformin, primarily recognized as a diabetes medication, contributes to weight management by enhancing insulin sensitivity and lowering blood sugar levels. Liraglutide, initially designed for diabetes treatment, is administered in lower doses for its GLP-1 mimicking effects, proving effective in supporting weight loss.
Ozempic, introduced in India by Novo Nordisk under the brand name Rybelsus in January 2022 for controlling blood glucose in adults with Type 2 diabetes, has also found utility among certain medical practitioners for its impact on hormones related to hunger, making it a consideration for weight loss. Additionally, doctors indicate ongoing trials for Wegovy and Mounjaro in India, exploring their potential as weight loss interventions. Despite their considerable benefits, GLP-1 analogs encounter significant challenges in terms of acceptance within the Indian market, primarily stemming from concerns related to pricing and the risk of hypoglycemia.
This paper aims to comprehensively explore and discuss the primary barriers hindering the early adoption of GLP-1 analogs in India, shedding light on the factors that contribute to these challenges and proposing potential strategies to address them.
Research Question
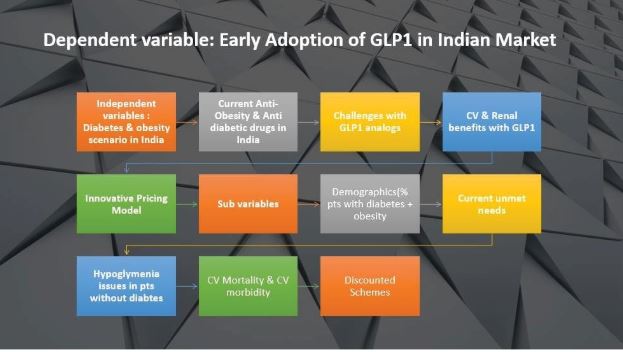
To comprehend the critical factors influencing the early adoption of GLP-1 in the Indian market, this study aims to identify and analyze the key dependent and independent variables involved.
Research Objectives
The primary objective of this study is to pinpoint the key constraints impeding the acceptance of Glucagon-like Peptide Antagonists in the management of diabetes and obesity. Furthermore, the study aims to devise an innovative strategy to effectively overcome these identified constraints.
Literature Review
Type 2 Diabetes Mellitus (T2DM) is a prevalent metabolic disorder resulting from a combination of factors: defective insulin secretion by pancreatic β-cells and the impaired response of insulin-sensitive tissues. The intricate molecular mechanisms governing insulin synthesis, release, and detection are crucial for maintaining glucose homeostasis, and any defects in these processes can lead to metabolic imbalances contributing to the development of T2DM.
The global market for type 2 diabetes drugs is anticipated to vary widely over the next decade, with projections reaching over US$100 billion for some medications [3]. By 2045, the overall global expenditure on diabetes-related health is estimated to rise to $1054 billion. The prevalence of obesity and abdominal obesity in India is significant, with older age, female gender, increased educational status, higher wealth index, marital status, and urban residency all contributing to elevated odds of obesity and abdominal obesity [4].
The wide variation in prices for oral anti-diabetic drugs in India poses a significant challenge to making medications more accessible and affordable to the general population. This price fluctuation plays a pivotal role in patient compliance, highlighting the importance of creating awareness about these disparities [5]. Additionally, the prevalence of obesity in India, currently at approximately 12% of the population, underscores the need for effective strategies to address both obesity and abdominal obesity [6].
The potential of GLP-1 receptor agonists in the treatment of type 2 diabetes, particularly their role in weight reduction. Semaglutide, administered orally in high doses, and the amylin analog cagrilintide, either alone or in combination with semaglutide injections, have demonstrated effectiveness in weight reduction. Tirzepatide, a GLP-1 receptor/GIP- R dual agonist, exhibits promising results in decreasing both glucose levels and body weight. Clinical trials for GLP-1R/GCG-R dual agonists and GLP-1R/GCG-R/ GIP-R triple agonists are underway [7].
Over the past two decades, GLP-1 receptor agonists have shown remarkable promise in treating people with type 2 diabetes. Their benefits extend beyond improved glycemic control to include weight loss, improved cardiovascular risk factors, and reductions in markers of fatty liver and renal disease. GLP-1 receptor agonists are considered an attractive therapeutic option due to their comprehensive positive effects [8].
With a focus on extra-glycemic effects such as weight loss, blood pressure reduction, and improved cholesterol levels, GLP-1 receptor agonists are recognized as calorie restriction mimetics or facilitators. Large clinical trials have also demonstrated cardiovascular risk reduction with these agonists. Economic evaluations of antidiabetic medications generally report good quality and use validated diabetes models. Newer antidiabetic medications have been found to be cost-effective compared to insulin, TZDs, and sulfonylureas [9].
Currently, GLP-1 agonists are licensed for use in adults and children over 10 years old by the US FDA for T2DM and for weight management in adults by the FDA and EMA. A meta-analysis supports the use of GLP-1 agonists in managing children with T2DM, demonstrating comparable effects on HbA1c and FPG as in adults (World ahead 2024).
In 2024, Novo Nordisk and Eli Lilly are poised to compete for dominance in the potentially $77 billion obesity treatment market by 2030 with their drugs Wegovy (semaglutide) and Mounjaro (tirzepatide). The market’s size has attracted considerable competition and innovation, with over 70 other obesity treatments in development. GLP-1 agonists, particularly glucagon-like peptide 1 (GLP-1) agonists, are a focal point, and their use is increasingly viewed as a medical necessity rather than a cosmetic concern. Recent studies on Wegovy have shown a 20% reduction in major cardiovascular events, positioning weight-loss injections as potential preventatives for hundreds of thousands of heart failures, both in America and globally. However, challenges related to patient psychology, likening drug dependence to an opiate addiction, are noted, and the broader acceptance of daily oral drugs for obesity treatment may require time.
Materials and Methods
Research Strategy and Eligibility Criteria: The research will embrace a pragmatic philosophy, aiming to take a practical viewpoint on various factors influencing constraints for the adoption of GLP1 analogs in India. The chosen research approach will be inductive, focusing on identifying barriers to the acceptance of newer molecules in the treatment of diabetes and obesity.
Data Extraction: To achieve comprehensive insights, a qualitative and quantitative mixed-methods approach will be employed. The study will incorporate a secondary literature review and employ other appropriate qualitative methods to identify and assess the major hurdles hindering the adoption of new therapies in the context of diabetes and obesity in India.
Quality Assessment and Statistical Analysis: The collected data will be subjected to a rigorous quality assessment process. The findings from these components will be utilized to formulate questionnaire items. This survey questionnaire will be administered to 53 healthcare practitioners responsible for managing diabetic and obesity populations in India. The data collected will be meticulously analyzed using Excel, employing both qualitative and quantitative techniques to derive meaningful insights.
Survey Questionnaire Diabetes & obesity in India
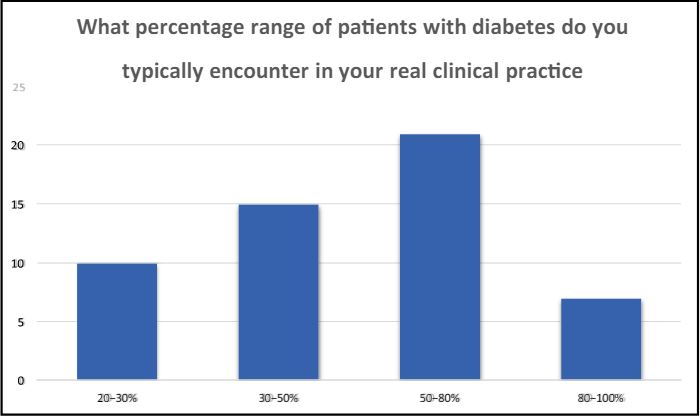
1 What percentage range of patients with diabetes do you typically encounter in your real clinical practice? A) 20- 30% B) 30-50% C) 50-80% D) 80-100%
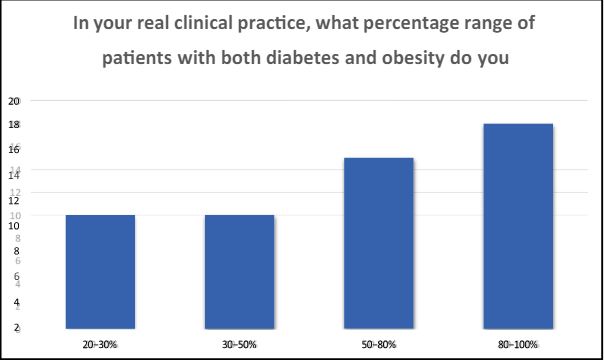
2 In your real clinical practice, what percentage range of patients with both diabetes and obesity do you typically encounter? A) 20-30% B) 30-50% C) 50-80% D) 80-100%
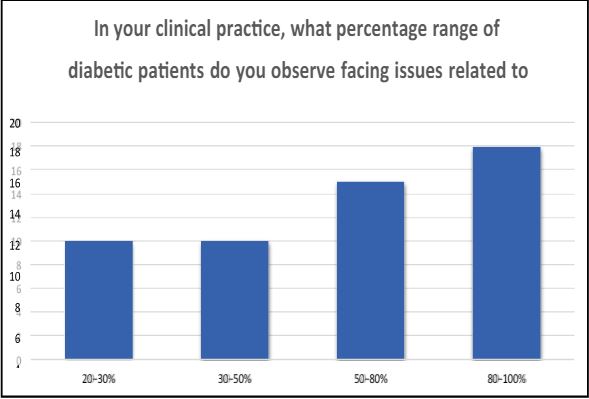
3 In your clinical practice, what percentage range of diabetic patients do you observe facing issues related to obesity? A) 20-30% B) 30-50% C) 50-80% D) 80-100%
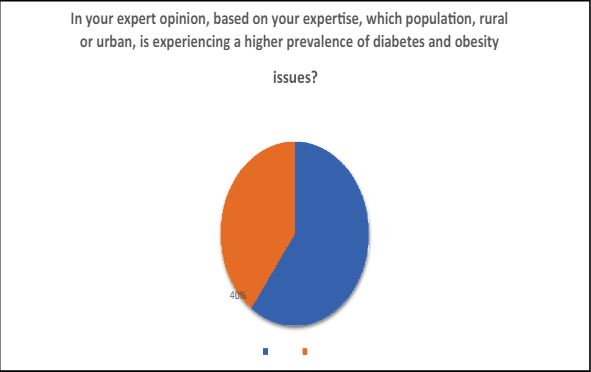
4 In your expert opinion, based on your expertise, which population, rural or urban, is experiencing a higher prevalence of diabetes and obesity issues?
Current Anti-Obesity & Anti diabetic drugs in India
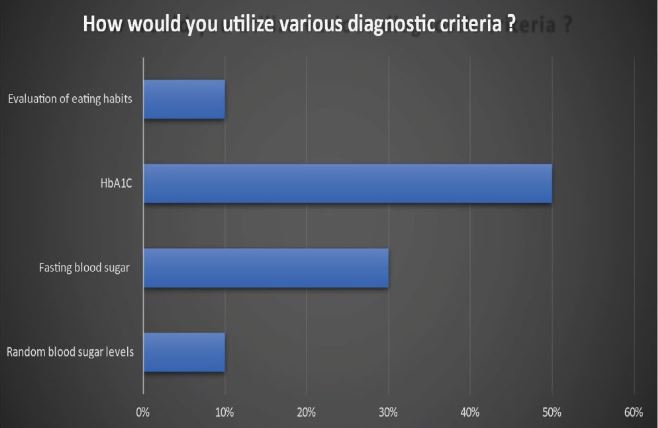
“How would you utilize various diagnostic criteria, including A) random blood sugar levels, B) fasting blood sugar, C) HbA1C levels, and D) an evaluation of eating habits, to assess and determine the presence of diabetes in a patient?
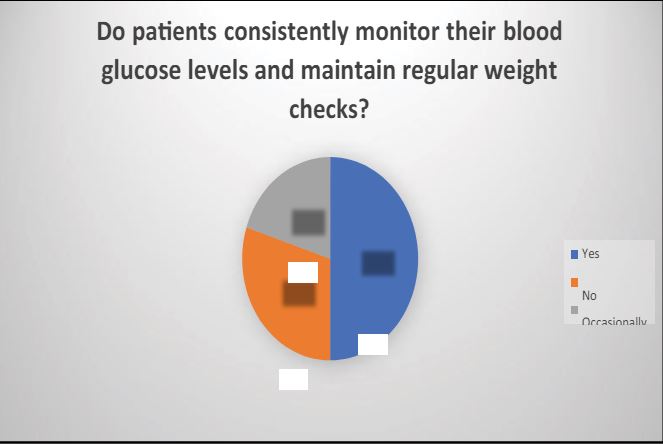
2 “Do patients consistently monitor their blood glucose levels and maintain regular weight checks? If so, how frequently do they engage in these monitoring activities: Yes, No, or occasionally?”
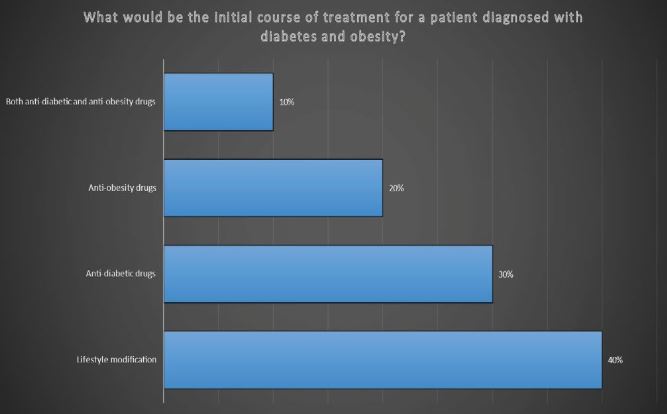
3 “When addressing diabetes and obesity in a diagnosed patient, which would be the primary treatment approach among the following options: a) Lifestyle modification b) Anti-diabetic drugs c ) Anti-obesity drugs d) Both anti-diabetic and anti-obesity drugs?”
Challenges with GLP1 analogs
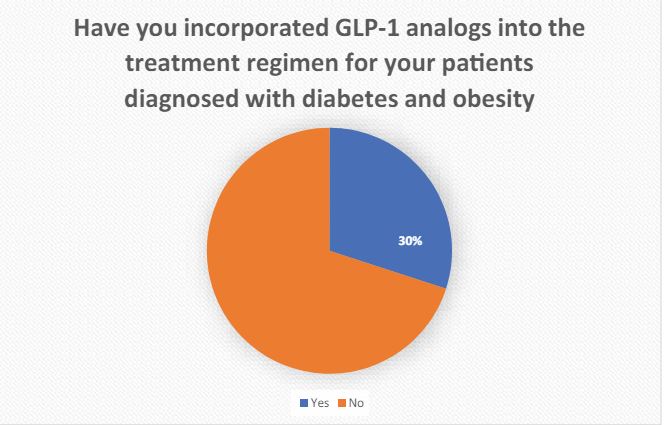
1 Have you incorporated GLP-1 analogs into the treatment regimen for your patients diagnosed with diabetes and obesity? Please respond with either ‘Yes’ or ‘No’.
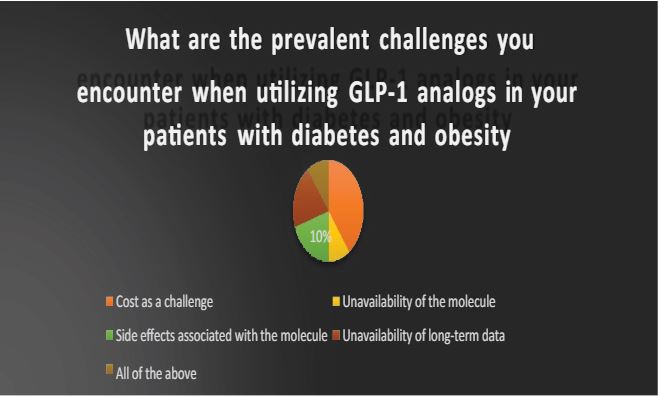
2 What are the prevalent challenges you encounter when utilizing GLP-1 analogs in your patients with diabetes and obesity? Select all that apply: A) Cost as a challenge B) Unavailability of the molecule C) Side effects associated with the molecule D) Unavailability of long-term data E) All of the above
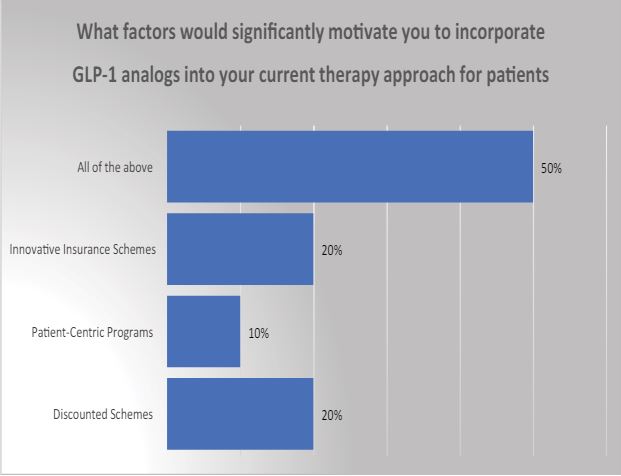
3 What factors would significantly motivate you to incorporate GLP-1 analogs into your current therapy approach for patients with obesity and diabetes? A) Discounted Schemes
B) Patient-Centric Programs C) Innovative Insurance Schemes D) All of the above Results
Diabetes & obesity in India
In the present landscape, healthcare professionals, whether specialized in diabetology, endocrinology, or general medicine, are increasingly managing a substantial percentage, ranging from 50% to 80% of their patient population grappling with diabetes. This underscores the pervasive nature of diabetes and emphasizes the critical role these healthcare providers play in addressing the growing prevalence of the condition.
Physicians find themselves consistently immersed in the care of patients, with an overwhelming majority ranging from 80% to 100%, who grapple with the challenges of both diabetes and obesity. This underscores the significant and prevalent burden of these health conditions, highlighting the crucial role physicians play in managing and addressing these complex medical concerns.
An overwhelming 95% of diabetic patients, on average, find themselves within the spectrum of being overweight or experiencing challenges related to obesity. This substantial correlation between diabetes and excess weight underscores the intricate interplay between these health conditions and emphasizes the need for comprehensive approaches to address both aspects of patients’ well-being.
The urban population is increasingly grappling with elevated rates of diabetes and obesity, primarily attributed to the prevalent sedentary lifestyle choices. This underscores the significant impact of modern, less active routines on health, highlighting the urgent need for lifestyle interventions to mitigate these prevalent health challenges.
Current Anti-Obesity & Anti diabetic drugs in India
The preferred method for diagnosing diabetes is assessing HbA1C levels, with Fasting Plasma Glucose (FPG) serving as a complementary measure. Furthermore, an in-depth consideration of dietary habits, fluid intake, and occasional random glucose checks assumes a comparatively lesser role when compared to the comprehensive insights provided by other biomarkers in the diagnostic process.
In the contemporary setting, a majority of individuals conscientiously monitor their blood glucose levels and weight routinely. However, certain individuals, constrained by demanding schedules, regrettably, find it challenging to consistently engage in the regular monitoring of their blood glucose and weight.
Challenges with GLP1 analogs
Lifestyle modification stands as the primary approach in the treatment of diabetes and obesity, with a focus on healthy habits and behaviors. Following this, anti-obesity and anti-diabetic medications may be introduced as part of a comprehensive treatment strategy.
GLP-1, with its potential benefits, emerges as a promising avenue for patients dealing with diabetes and obesity. However, the early adoption of GLP-1 in current medical practice faces numerous challenges.
The broad acceptance of GLP-1 for diabetic patients contending with obesity in India faces substantial challenges, primarily due to cost considerations. Moreover, apprehensions surrounding potential side effects and the limited availability of long-term data on cardiovascular and renal safety significantly influence the early adoption of this molecule.
Addressing the cost challenge associated with the acceptance of GLP-1 in India could be mitigated if multinational corporations (MNCs) introduce comprehensive insurance schemes, sophisticated patient-centric programs, and discounted initiatives. Such measures may enhance the appeal of these new launches among physicians, prompting consideration for a broader range of diabetic and obese patients.
Conclusion
Diabetes mellitus (DM) presents a multifaceted and progressive health challenge, elevating the risk of complications for affected individuals. Weight gain, a common occurrence in type 2 DM, not only complicates treatment but also increases the cardiovascular risk for patients. Current therapies often fall short in addressing key aspects of type 2 diabetes management, such as the progressive decline in beta-cell function, dysregulated glucagon release, reduced incretin effect, and associated weight gain.
In response to these unmet needs, GLP-1 analogues have emerged as promising solutions, providing a comprehensive approach to diabetes management. These analogues effectively address various issues by inducing glucosedependent insulin release, inhibiting glucagon release, delaying gastric emptying, enhancing satiety, reducing energy intake, and improving insulin sensitivity. As a result, GLP-1 analogues offer an appealing treatment option to effectively manage diabetes.
The primary objective of our research is to gain a profound understanding of the critical factors influencing the early adoption of GLP-1 in the Indian market. This study is designed to identify and analyze both dependent and independent variables that play a crucial role in this adoption process. Our focus is to pinpoint key constraints hindering the acceptance of Glucagon-like Peptide Antagonists in the management of diabetes and obesity, and subsequently, devise an innovative strategy to overcome these identified constraints.
To ensure a comprehensive exploration of these factors, we will employ a mixed-methods approach, incorporating both qualitative and quantitative methodologies. The research plan includes a thorough secondary literature review and the utilization of appropriate qualitative methods to identify and assess major hurdles hindering the adoption of new therapies in the context of diabetes and obesity in India. A carefully designed survey questionnaire will be administered to 53 healthcare practitioners responsible for managing diabetic and obesity populations in India. The collected data will undergo meticulous analysis using Excel, employing both qualitative and quantitative techniques to extract meaningful insights.
In the current healthcare landscape, professionals across various medical disciplines, including diabetology, endocrinology, and general medicine, are increasingly managing a significant percentage of patients dealing with diabetes, ranging from 50% to 80%. Notably, a substantial 95% of diabetic patients experience challenges related to being overweight or obese. The urban population, in particular, faces elevated rates of diabetes and obesity due to prevalent sedentary lifestyles.
The preferred diagnostic approach involves assessing HbA1C levels, complemented by Fasting Plasma Glucose (FPG) measurements. Routine monitoring of blood glucose levels and weight is a common practice among individuals. Lifestyle modification is emphasized as the primary approach in treating diabetes and obesity, focusing on cultivating healthy habits and behaviors. While GLP-1 holds promise for patients grappling with diabetes and obesity, its early adoption in current medical practice encounters challenges.
The widespread acceptance of GLP-1 in diabetic patients dealing with obesity in India is hindered primarily by cost considerations. Additionally, concerns regarding potential side effects and the limited availability of long-term cardiovascular and renal safety data significantly impact early adoption. To address the cost challenge associated with GLP-1 acceptance in India, multinational corporations (MNCs) could introduce comprehensive insurance schemes, patient-centric programs, and discounted initiatives. These measures have the potential to enhance the appeal of new launches among physicians, fostering consideration for a broader range of diabetic and obese patients.
Future Research
In 2023, GLP-1 medications took center stage in the healthcare landscape, sparking significant interest and discussions. The momentum around these medications is anticipated to grow, prompting leading companies to reconsider their selling approaches. The heightened focus on weight-loss medications, particularly GLP-1s, underscores the need for companies to adapt to evolving trends. However, the soaring costs of these drugs have raised concerns among patients, employers, and payers, necessitating a reevaluation of selling strategies.
Business-to-consumer GLP-1 companies, now targeting the employer space, claim to possess the ultimate solution to managing GLP-1 expenditure. This dynamic landscape signals a crucial juncture for multinational corporations (MNCs) to meticulously design effective brand strategies for upcoming launches, such as tirzepatide. Notably, this compound has demonstrated remarkable results, aiding overweight or obese adults in achieving an average 20% reduction in mean body weight.
Despite these promising outcomes, healthcare professionals (HCPs) remain somewhat hesitant to fully embrace the molecule for effective weight loss and diabetes control. This hesitancy stems from factors such as the high cost, the risk of hypoglycemia in nondiabetic patients, and a perceived lack of comprehensive cardiovascular and renal safety data. Addressing these concerns is paramount, necessitating a concerted effort to conduct additional studies that not only establish the real need for the molecule but also provide robust evidence regarding its safety and efficacy.
To enhance the acceptance of GLP1, MNCs should pioneer innovative pricing models that balance affordability with the substantial benefits offered by these drugs. Defining a sustainable goal to establish the efficacy and safety of the molecule among physicians is crucial. This involves not only showcasing the weight-loss potential but also addressing concerns related to hypoglycemia and ensuring comprehensive data on cardiovascular and renal safety
In summary, the growing prominence of GLP-1 medications, demands a strategic reevaluation of selling approaches by MNCs. The focus should extend beyond the initial successes and address lingering concerns among healthcare professionals, ultimately fostering widespread acceptance and utilization of these innovative therapies.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, et al. (2012) Management of hyperglycemia in type 2 diabetes: a patientcentered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Spectrum 25: 154-171.[ Ref ]
Drucker DJ (2022) GLP-1 physiology informs the pharmacotherapy of obesity. Molecular Metabolism 57: 101351.[ Ref ]
Lancet T (2023) Diabetes: a defining disease of the 21st century. Lancet (London, England) 401: 2087.[ Ref ]
Gupta RD, Tamanna N, Siddika N, Haider SS, Apu EH, et al. (2023) Obesity and Abdominal Obesity in Indian Population: Findings from a Nationally Representative Study of 698,286 Participants. Epidemiologia 4: 163-172.[ Ref ]
Wadagbalkar P, Bhadoriya SS (2023) Pharmacoecomonic study of oral antidiabetic drugs available in Indian pharmaceutical market. International Journal of Basic & Clinical Pharmacology 12: 677-682.[ Ref ]
Chakhtoura M, Haber R, Ghezzawi M, Rhayem C, Tcheroyan R, et al. (2023) Pharmacotherapy of obesity: An update on the available medications and drugs under investigation. EClinicalMedicine 58: 101882.[ Ref ]
Bailey CJ, Flatt PR, Conlon JM (2023) An update on peptide-based therapies for type 2 diabetes and obesity. Peptides 161:170939.[ Ref ]
Williams DM, Staff M, Bain SC, Min T (2022) Glucagon-like peptide-1 receptor analogues for the treatment of obesity. Touch REVIEWS in Endocrinology 18: 43.[ Ref ]
Hong D, Jiang M, Si L, Shao H, Ming WK, et al. (2019) Cost effectiveness of sodium-glucose cotransporter-2 (SGLT2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and dipeptidyl peptidase-4 (DPP4) inhibitors: a systematic review. Pharmacoeconomics 37: 777-818.[ Ref ]