Journal Name: Journal of Health Science and Development
Article Type: Research
Received date: 02 March, 2021
Accepted date: 19 May, 2021
Published date: 2024-02-01
Citation: Zhao H, He W, Li X, Zhang S, Liu C, et al. (2021) Serum Interleukin-18 Levels are not increased in Type 2 Diabetic Patients. J Health Sci Dev Vol: 4, Issu: 1 (16-20).
Copyright: © 2021 Zhao H et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: To investigate the effects of interleukin 18 (IL-18) on diabetic retinopathy (DR) of type 2 diabetic patients, the contents of IL- 18 were measured in serum of 206 case subjects with type 2 diabetes and 40 case subjects without diabetes as control. Methods: According to the degree of DR, the diabetic patients were further divided into three groups: non-diabetic retinopathy (NDR, n=69), non-proliferative diabetic retinopathy (NPDR, n = 52) and proliferative diabetic retinopathy (PDR, n=85). Results: Unlike previous reports, we didn’t find a significant increase of serum IL-18 in diabetic patients (mean ± SD are 107.4±36.6 and 112.5±32.0 pg/ml for control and type 2 diabetes patients respectively, p > 0.05). Furthermore, we also failed to find any significant increase of serum IL-18 in patients with NDR, NPDR or PDR (113.0±32.1, 110.8±31.4 and 114.5±33.4 pg/ml respectively) compared with the control group (for all values, p > 0.05). The results of real-time Polymerase Chain Reaction (PCR) showed that there was no significant difference in the expression of IL-18 mRNA between type 2 diabetic patients with DR and the control group (P > 0.05). Interestingly, there was a significant positive correlation between the levels of serum IL-18 and the amount of fasting blood glucose (FBG, r=0.15, p=0.03). Hemoglobin A1c (HbA1c) was relatively higher in diabetic patients compared to patients in control subjects (p<0.05). These results suggest that the levels of serum IL-18 in diabetic patients are within the normal range. Even in patients with diabetic retinopathy, the levels of serum IL -18 were only slightly increased in type 2 diabetic patients, and there were no difference from control subjects. Conclusion: these data suggest that the serum IL-18 levels are not associated with the severity of type 2 diabetic patients.
Keywords:
Interleukin 18, Type 2 diabetic patients, Diabetic retinopathy.
Abstract
Background: To investigate the effects of interleukin 18 (IL-18) on diabetic retinopathy (DR) of type 2 diabetic patients, the contents of IL- 18 were measured in serum of 206 case subjects with type 2 diabetes and 40 case subjects without diabetes as control. Methods: According to the degree of DR, the diabetic patients were further divided into three groups: non-diabetic retinopathy (NDR, n=69), non-proliferative diabetic retinopathy (NPDR, n = 52) and proliferative diabetic retinopathy (PDR, n=85). Results: Unlike previous reports, we didn’t find a significant increase of serum IL-18 in diabetic patients (mean ± SD are 107.4±36.6 and 112.5±32.0 pg/ml for control and type 2 diabetes patients respectively, p > 0.05). Furthermore, we also failed to find any significant increase of serum IL-18 in patients with NDR, NPDR or PDR (113.0±32.1, 110.8±31.4 and 114.5±33.4 pg/ml respectively) compared with the control group (for all values, p > 0.05). The results of real-time Polymerase Chain Reaction (PCR) showed that there was no significant difference in the expression of IL-18 mRNA between type 2 diabetic patients with DR and the control group (P > 0.05). Interestingly, there was a significant positive correlation between the levels of serum IL-18 and the amount of fasting blood glucose (FBG, r=0.15, p=0.03). Hemoglobin A1c (HbA1c) was relatively higher in diabetic patients compared to patients in control subjects (p<0.05). These results suggest that the levels of serum IL-18 in diabetic patients are within the normal range. Even in patients with diabetic retinopathy, the levels of serum IL -18 were only slightly increased in type 2 diabetic patients, and there were no difference from control subjects. Conclusion: these data suggest that the serum IL-18 levels are not associated with the severity of type 2 diabetic patients.
Keywords:
Interleukin 18, Type 2 diabetic patients, Diabetic retinopathy.
Introduction
Recently, there is a great debate about the effect of Interleukin 18 (IL-18) on the retinopathy including diabetic retinopathy (DR) and age-related macular degeneration (AMD). IL-18, as proinflammatory cytokines, is a member of IL-1 cytokine superfamily and plays an important role in immune regulation and immune pathological lesion. Serum IL-18 levels were up-regulated in a variety of inflammatory diseases and acute injury such as Acute Kidney Injury, Acute hepatic necrosis, Rheumatoid Arthritis and so on [1-3]. In the past decade, it has been reported that the serum IL-18 levels were increased in type 1, 2 diabetic patients with retinopathy and speculated that IL-18 may be associated with the pathogenesis of DR [4-6]. However, bioactivity assay indicates that IL-18 not only inhibits the formation of new blood vessels but also promotes the maturity of new blood vessels in vivo and in vitro [7-10]. Since then, the role of IL-18 in DR and AMD has drawn a great amount of attention. One interesting function of IL-18 is that it specifically inhibits the proliferation of capillary endothelial cell and neovascularization in cornea [11]. IL- 18 gene knockout results in retinal vascular expansion and leakage, as well as overexpression of vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), bFGF, and pigment epithelium-derived factor (PEDF) in newborn mice, suggesting that IL-18 has an inhibitory role in choroidal neovascularization (CNV) [12]. Animal experiments have shown that IL-18 inhibits angiogenesis and vascular leakage by suppressing the activity of VEGF. In a mice CNV model, intraocular injection of VEGF and VEGF caused obvious retinal vascular leakage, while the mice received recombinant IL-18 led to a significant reduction in the amount of neovascularization [13,14]. A recent study suggests that recombinant human IL-18 is safe and can reduce the development of choroidal neovascular lesion in cynomolgus monkeys [7]. However, the other studies have shown that serum IL-18 level was up- regulated in type 2 diabetic patients. This is consistent with reports revealing that IL-18 has no therapeutic effects on neovascular AMD [15]. Surprisedly, our results showed that serum IL-18 levels in type 2 diabetic patients were not significantly increased. The present study analyzed the expression level of IL-18 in patients with type 2 diabetic retinopathy to investigate the safety of IL-18 in the treatment of diabetic retinopathy.
Materials and Methods
Subjects
In total, 246 subjects consisting of 206 patients with type 2 DR were used in the current study. Patients with diseases like acute infection, hypertension, atherosclerotic heart disease, hypothyroidism and malignancy were excluded. Subjects in the control group were not receiving any medication. Type 2 DR was diagnosed according to WHO standard in 1999. The diabetic patients were further divided into three groups according to diabetic retinopathy lesions: non-diabetic retinopathy (NDR, n=69), non-proliferative diabetic retinopathy (NPDR, n = 52), proliferative diabetic retinopathy (PDR, n = 85). All of the subject characteristics were summarized in table 1.
Table 1:Clinical and biochemical features of the subjects.
| Control group (n = 40) | Diabetic patients (n = 206) | p | |
|---|---|---|---|
| Age(years) | 61.5±5.0 | 57.8±7.9 | 0.861 |
| Duration of diabetes (years) | - | 9.4±5.5 | - |
| FBG(mmol/L) | 5.6±0.9 | 9.7±2.7 | 0.021 |
| HbA1c (%) | 5.2±0.7 | 8.1±1.8 | 0.026 |
| AGEs | 0.1±0.04 | 0.3±0.06 | 0.041 |
| IL-18 (pg/ml) | 107.4±36.6 | 112.5±32.0 | 0.463 |
| FBG: fasting blood glucose; AGEs: spontaneous fluorescence value; IL-18: interleukin-18. | |||
Methods
Fasting blood glucose (FBG), Hemoglobin A1c (HbA1c), and spontaneous fluorescence value (AGEs) levels. For measuring IL-18, samples were centrifuged and stored at -80ºC until the day of analysis. Before the day of analysis, serum samples were transferred to -20ºC and were dissolved at room temperature.
Quantification of IL-18 with Enzyme-linked immunosorbent assay (ELISA)
Serum IL-18 levels were measured with ELISA kit (TaKaRa Biotechnology, Shenyang, China). Results were expressed as pg/ml.
Real-time quantitative PCR
The expression of IL-18 messenger RNA (mRNA) was examined by real-time quantitative PCR detecting system (qPCR). Total RNAs were separated from people peripheral blood using RNAiso reagent (TaKaRa Biotechnology, Shenyang, China). The total RNAs were treated with DNase I (TaKaRa Biotechnology, Shenyang, China) and then subjected to reverse transcription using PrimeScript™ RT Reagent Kit (Perfect Real Time) (TaKaRa Biotechnology, Shenyang, China). The qPCR experiments were performed with a TaKaRa TP800Real Time PCR System (TaKaRa Biotechnology, Shenyang, China) using 2μl cDNA with 10μl SYBR green PCR mastermix (TaKaRa Biotechnology, Shenyang, China) and 0.4μl of each specific primer. The glyceraldehyde-3-phosphatedehydrogenase (GAPDH) of human was used as an internal control to normalize the starting quantity of RNA.
Statistical analysis
All statistical analyses were performed using IBM SPSS statistics software (version 22; SPSS). Data were analyzed using parametric statistics with two-tailed Student’s test, bivariate correlation test, or X2 –Test, as appropriate. All data are expressed as mean ± SEM.
Results
Serum IL -18 levels are comparable between diabetic patients and control subjects
The clinical and biochemical features of the subjects included in the study are summarized in Table 1. The levels of FBG, HbA1c and AGEs in type 2 DR were significantly higher than those in control group. However, the levels of serum IL-18 were comparable between type 2 DR patients and control subjects (table 1). It can be concluded that the IL-18 levels was security treatment in type 2 DR.
Diabetic retinopathy was related to IL-18 levels
Type 2 diabetes patients were divided into three groups according to diabetic retinopathy lesions: NDR, NPDR, and PDR. As shown in table 2, the expression levels of IL-18 in three groups of type 2 DR were slightly higher than those in the control group, but there was no significant difference. Thus, the level of serum IL-18 was not associated with the severity of type 2 diabetes.
Diabetic retinopathy was related to IL-18 levels
Type 2 diabetes patients were further divided into four groups according to duration of diabetes: 0-5years, 5-10years, 10-15years,> 15years. As shown in table 3 the duration of diabetes was not associated with the security of type 2 DR.
The relative expression of IL-18 mRNA
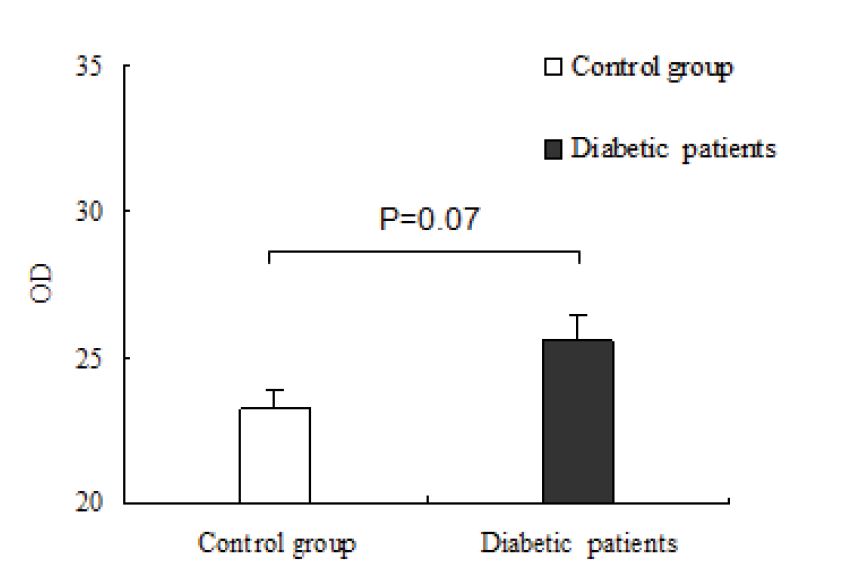
The IL-18 mRNA levels were examined by a Real-time quantitative PCR detecting system (qPCR). The IL-18 mRNA level was detected in 4 control subjects and 7 type 2 DR patients. As shown in figure 1, the expression of IL-18 was slightly upregulated in diabetes patients, but there was not significant difference compared with the control group (p=0.07). Type 2 diabetes patients were divided into three groups according to serum IL-18 levels: ≤ 80 pg/ml, 80-120 pg/ml, and > 120 pg/ml. With the increase of IL–18 levels, the patient’s vision did not change significantly. Thus, the vision of diabetic patients was not affected by the serum IL- 18 levels. From the above results, it can be inferred that IL- 18 plays an important role in security treatment and has no side effects.
Table 2:The serum IL-18 levels of diabetic retinopathy.
| Control group (n = 40) | Diabetic patients | |||
|---|---|---|---|---|
| NDR (n = 69) | NPDR (n = 52) | PDR (N=85) | ||
| IL-18 (pg/ml) | 107.4±36.6 | 113.0±32.1 | 110.8±31.4 | 114.5±33.4 |
| p | 0.897 | 0.428 | 0.608 | |
| p values were obtained from t test between diabetic patients and control subjects | ||||
Table 3:The serum IL-18 levels of duration of diabetes.
| Control group (n = 40) | Diabetic patients | (Years) | |||
|---|---|---|---|---|---|
| ≦5 (n = 53) | 5-10 (n = 94) | 10-15 (n = 25) | ﹥15 (n = 31) | ||
| IL-18 (pg/ml) | 107.4±36.6 | 113.0±31.1 | 113.6±33.1 | 109±30.7 | 112.7±33.5 |
| p | 0.341 | 0.266 | 0.942 | 0.873 | |
| p values were obtained from t test between diabetic patients and control subjects | |||||
FBG was related to IL-18 levels
In diabetic patients, we found that there was a significant correlation between the levels of serum IL18 and FBG (r =0.15, p = 0.03), and then HbA1c (r = 0.15, p = 0.02) was relatively high. These results suggest that the expression of IL-18 in normal human and diabetic patients was within the normal range. Although the level of IL-18 increased slightly with the aggravation of fundus lesions, the difference was not statistically significant. Thus, IL-18 level and type 2 DR were no correlation.
Discussion
With the extension of life expectancy of Chinese population and the changes in dietary habits and structure, the incidence of diabetes is increasing year after year. According to the latest statistics, currently about 50 million people are facing the threat of diabetes in China, and diabetes has become a common disease affecting human health. In addition, DR is one of the leading causes of blindness. The pathogenesis of type 2 DR is still unclear, thus the study of pathogenesis, prevention and therapy of DR has become urgent in medical science. The cytokine plays an important role in mediated inflammatory mechanisms in type 2 diabetic patients. The studies have shown that IL-6, TNF-α and other proinflammatory cytokine levels are significantly increased in type 2 diabetic patients, suggesting that these proinflammatory cytokines have promoted inflammatory response in the pathogenesis of type 2 diabetic patients [16]. IL-18 is a multifunctional cytokine in the inflammatory cascade and is critical in promoting inflammation pathogenesis in type 2 diabetic patients [17]. Multiple reports have indicated that the serum IL-18 levels in type 2 diabetic patients were elevated in varying degrees [18]. In contrast, there are studies showing that in AMD patients, the IL-18 level in the aqueous humor was decreased and that the IL-18 level was positively correlated with vision and the therapeutic efficacy of monoclonal antibody. Thus, the increase of serum IL-18 level in type 2 diabetic patients and its role in the pathogenesis of DR is still a debate.
The serum IL-18 mRNA expression levels in control and diabetic patients.
The severity of diabetes was not related to the change of IL-18 level
The current study results show that the levels of serum IL-18 from type 2 diabetic patients with different degrees of diabetes were slightly higher compared to the control group (Table 1 and Figure 1). The further analysis of serum IL-18 levels in patients with diabetes revealed that the severity of diabetes was also not associated with the change in IL- 18 levels (Table 2). In addition, there were no significant correlations between the serum IL-18 levels and medical history as well as vision among diabetic patients.
IL-18 mRNA was not significantly increased in patients with type 2 diabetes mellitus
As an endogenous multifunctional regulator cytokine, IL- 18 is first synthesized into a precursor protein Pro-IL-18 in cells, which is then digested by caspase-1 to become active mature IL-18 [19]. However, partial of IL-18 is inhibited by IL-18 binding protein. In normal circumstances the total mature IL-18 levels in blood is about 80-120 pg/mL [20]. However, ELISA only detects the free IL-18. To reflect the expression level of IL-18 in type 2 diabetic patients, the expression of IL-18 mRNA in peripheral blood cells was measured by quantitative PCR (RT qPCR). It was found that the expression of IL-18 mRNA was no significant increase compared with normal control subjects (Figure 1).
IL-18 plays a critical role in inhibiting the formation of new blood vessels
In a CNV model, VEGF and VEGF injected into the eyes can cause obvious retinal vascular leakage, while the area of neovascularization and infiltration area of mice injected with recombinant IL-18 are significantly reduced [13,14]. The latest studies suggest that recombinant human IL-18 is safe and can the development of choroidal neovascularization in cynomolgus monkeys [7]. Therefore, IL-18 plays a critical role in inhibiting angiogenesis. This study suggests that there is no significant difference between the serum IL-18 levels of diabetic patients and the control group by ELISA and RT-PCR, suggesting that the role of IL-18 in DR needs to be further studied. The capability of IL-18 to inhibit angiogenesis indicates that IL-18 is a good candidate for DR treatment.
Conclusion
In conclusion, we found that the serum IL-18 levels were not significantly increased in the patients with type 2 diabetic patients. The phase II clinical trials have proved that the recombinant IL-18 is safe in human subjects. The research results from the study of recombinant IL-18 in the Department of Ophthalmology have indicated that IL-18 has therapeutic value in pathological neovascularization.
Ethical Approval and Consent to Participate
The serum of patients with diabetes mellitus in this study was from the hospital directly under the group (He Eye Specialist Hospital), and the patients were informed and agreed, and were approved by the ethics committee.
Consent for Publication
All authors have read and approved the final manuscript.
Availability of Supporting Data
Not applicable.
Competing Interests
The authors declare that there are no competing interests.
Funding
This work was supported by the Science and technology foundation of Liaoning Province (2015010568-301).
Authors’ Contributions
All corresponding and first authors contributed to the study concept and design.SZ and CL wrote the manuscript. ZW performed the experiments PZ analyzed the data. All authors reviewed and approved the final manuscript.
Acknowledgement
Not applicable
Duangporn TN, Pisit T, Rungsun L, Mahachai V, Theamboonlers A, et al. (2006) Diagnostic role of serum interleukin-18 in gastric cancer Patients. World J Gastroenterol 12: 4473-4477. [ Ref ]
Yang Y, Qiao J, Li R (2011) Is interleukin-18 associated with polycystic ovary syndrome? Reproductive Biology and Endocrinology 9: 7. [ Ref ]
Shen J, Choy DF, Yoshida T, Iwase T, Hafiz G, et al. (2014) Interleukin-18 has antipermeablity and antiangiogenic activities in the eye: reciprocal suppression with VEGF. Cell Physiol 229: 974-983. [ Ref ]
Alev EA, Ilhan Y, Esen A, Bukan N, Arslan M (2008) SerumIL-18 levels in patients with type 1 diabetes:Relations to metabolic control and microvascular complications. Cytokine 42: 217-221. [ Ref ]
Katakami N, Yamamoto PK, Hideaki K, Yoshiuchi K, Kato K, et al. (2007) Serum Interleukin-18 Levels Are Increased and Closely Associated With Various Soluble Adhesion Molecule Levels in Type1 Diabetic Patients. Diabetes Care 30: 158-161. [ Ref ]
Song Z, Sun M, Zhou F, Huang F, Qu J, et al. (2014) Increased intravitreous interleukin-18 correlated to vascular endothelial growth factor in patients with active proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 252: 1229-1234. [ Ref ]
Doyle SL, López FJ, Celkova L, Brennan K, Mulfaul K, et al. (2015) IL- 18 Immunotherapy for Neovascular AMD: Tolerability and Efficacy in Nonhuman PrimatesIL-18 Immunotherapy for Neovascular AMD.Investigative ophthalmology & visual science 56: 5424-5430. [ Ref ]
Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, et al. (2003) Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA 289: 1799-1804. [ Ref ]
Escobar-Morreale HF, Botella-Carretero JI, Villuendas G, Sancho J, San Millán JL (2004) Serum interleukin-18 concentrations are increased in the polycystic ovary syndrome: relationship to insulin resistance and to obesity. The Journal of Clinical Endocrinology & Metabolism 89: 806-811. [ Ref ]
Moriwaki Y, Yamamoto T, Shibutani Y, Aoki E, Tsutsumi Z, et al. (2003) Elevated levels of interleukin-18 and tumor necrosis factor-α in serum of patients with type 2 diabetes mellitus: relationship with diabetic nephropathy. Metabolism 52: 605-608. [ Ref ]
Renhai C, Jacob F, Masashi K, Yihai C (1999) Iterleukin-18 acts as an angiogenes is and tumor suppressor. The FASEB Journal 13: 2195- 2202. [ Ref ]
Qiao H, Sonoda KH, Sassa Y, Toshio H, Hiroshi Y, et al. (2004) Abnormal retinal vascular development in IL-18 knockout mice. Lab Invest 84: 973-980. [ Ref ]
Shen J, Choy DF, yoshida T, Iwase T, Hafiz G, et al. (2014) Interleukin-18 has antipermeablity and antiangiogenic activities in the eye: reciprocal suppression with VEGF. J Cell Physiol 229: 974-983. [ Ref ]
Doyle SL, Ozaki E, Brennan K, Humphries MM, Mulfaul K, et al. (2014) IL-18 attenuates experimental choroidal neovascularization as a potential therapy for wet age-related macular degeneration. Sci Transl Med 6: 230. [ Ref ]
TaralloV, Hirano Y, Gelfand BD, Dridi S, Kerur N, et al. (2012) DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell 149: 847-859. [ Ref ]
Ziccardi P, Nappo F, Giugliano G, Esposito K, Marfella R, et al. (2002) Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation 105: 804-809. [ Ref ]
Gracie JA, Robertson SE, McInnes IB (2003) Interleukin-18. Journal of leukocyte biology 73: 213-224. [ Ref ]
Giunti S, Tesch GH, Pinach S, Burt DJ, Cooper ME, et al. (2008) Monocyte chemoattractant protein-1 has prosclerotic effects both in a mouse model of experimental diabetes and in vitro in human mesangial cells. Diabetologia 51: 198-207. [ Ref ]
LiuB, Novick D (2000) Production of a biologically active human interleukin 18 requires its prior synthesis as PRO-IL-18. Cytokine 12: 1519-1525. [ Ref ]
Novick D, Schwartsburd B, Pinkus R, Suissa D, Belzer I, et al. (2001) A novel IL-18BP ELISA shows elevated serum IL-18BP in sepsis and extensive decrease of free IL-18. Cytokine 14: 334-342. [ Ref ]