Journal Name: Journal of Health Science and Development
Article Type: Conceptual
Received date: 03 May, 2021
Accepted date: 16 July, 2021
Published date: 2024-02-01
Citation: Burgos-Salcedo J, Sierra C, Bunyard P (2021) Systems Immunology Approach of Within-host Dynamics of Coronavirus (SARS CoV-2) with Innate Immune Response. J Health Sci Dev Vol: 4, Issu: 1 (05-11).
Copyright: 2021 Burgos-Salcedo J et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Mathematical models are being used extensively in the study of SARS-CoV-2 transmission dynamics, becoming an essential tool for decision-making concerning disease control. It is now required to understand the mechanisms involved in the interaction between the virus and the immune response effector cells, both innate and adaptive, to support lines of research related to the use of drugs, production of protective antibodies, and actually, the following up of the dynamics of the pandemic as a result of vaccination trials against SARS-CoV-2 all around the world. In the present study, using a within-host dynamic approach, we hypothesize over the conditions that characterize the fraction of the population which get infected by SARS-CoV-2 as the asymptomatic patients, the mild symptomatic, acute symptomatic, in terms of the innate immune response, the initial virus load, and the cytokine levels, finding that the combination of high viral loads with deficient innate immune response led to acute or severe symptomatic COVID-19 patients, characterized by suffering viral shedding episodes frequently and with an increasing concentration of circulating cytokines leading to the cytokine storm.
Keywords:
Within-host SARS-CoV-2 modelling, Systems immunology, Virus load, Cytokine storm, COVID-19, Innate immunity.
Abstract
Mathematical models are being used extensively in the study of SARS-CoV-2 transmission dynamics, becoming an essential tool for decision-making concerning disease control. It is now required to understand the mechanisms involved in the interaction between the virus and the immune response effector cells, both innate and adaptive, to support lines of research related to the use of drugs, production of protective antibodies, and actually, the following up of the dynamics of the pandemic as a result of vaccination trials against SARS-CoV-2 all around the world. In the present study, using a within-host dynamic approach, we hypothesize over the conditions that characterize the fraction of the population which get infected by SARS-CoV-2 as the asymptomatic patients, the mild symptomatic, acute symptomatic, in terms of the innate immune response, the initial virus load, and the cytokine levels, finding that the combination of high viral loads with deficient innate immune response led to acute or severe symptomatic COVID-19 patients, characterized by suffering viral shedding episodes frequently and with an increasing concentration of circulating cytokines leading to the cytokine storm.
Keywords:
Within-host SARS-CoV-2 modelling, Systems immunology, Virus load, Cytokine storm, COVID-19, Innate immunity.
Introduction
COVID-19 pandemic has underlined the impact of emergent pathogens as a major threat to human health [1,2]. The development of quantitative approaches to advance comprehension of the current outbreak is urgently needed to tackle this severe disease [3]. Coronaviruses (CoV) are a broad family of viruses that can cause a variety of conditions, from the common cold to more serious illnesses, such as the coronavirus that causes Middle East respiratory syndrome (MERS-CoV) and the one that causes the actual respiratory syndrome acute severe (SARS-CoV-2), a new coronavirus that has not been found before in humans [1].
When an infected person expels virus-laden droplets and someone else inhales them, the novel coronavirus, SARS-CoV-2, enters the nose and throat, and given that the virus binds with a cell-surface receptor called angiotensin-converting enzyme 2 (ACE2), which is distributed throughout the entire body, the virus can disseminate into, potentially, all organs and tissues. As the virus multiplies, an infected person may shed copious amounts of it, especially during the first week or so. Symptoms may be absent at this point. Or the virus’ new victim may develop a fever, dry cough, sore throat, loss of smell and taste, or head and body aches. If the immune system doesn’t beat back SARS-CoV-2 during this initial phase, the virus then marches down the windpipe to attack the lungs, where it can turn deadly. The thinner, distant branches of the lung’s respiratory tree end in tiny air sacs called alveoli, each lined by a single layer of cells that are also rich in ACE2 receptors [4].
This series of events can be explained by taking into account that during an infection, the innate immunity is the first to be triggered (the inflammatory reaction), taking no longer than minutes to hours to be fully activated [5]. This is crucial for the host defense in the first phase of a new infection, and plays a central role in the response against SARS-CoV-2, which can explain why most of the infected people will never develop COVID-19 or present mild symptoms (80.9%). The innate immunity is generally able to eliminate pathogens efficiently, but if this initial clearance of SARS-CoV-2 fails, probably due to a high of virus load or virulence of invading pathogens; lymphocytes and adaptative immune mechanisms are activated, increasing antibodysecreting cells (ASCs), follicular helper T cells (TFH cells), activated CD4+ T cells and CD8+ T cells and immunoglobulin M (IgM) and IgG antibodies that bound the COVID-19 causing coronavirus SARS-CoV-2 were detected in blood before symptomatic recovery. Also Accumulating evidence suggests that a subgroup of patients with severe COVID-19 might have a cytokine storm syndrome. However, 13.8% of the infected people develop severe symptoms, 4.4% become critically ill and 2.9% die. These patients usually had chronic diseases, including cardiovascular and cerebrovascular diseases, endocrine system disease, digestive system disease, respiratory system disease, malignant tumors, and nervous system disease [6].
Regarding the immune response against the SARS CoV2 virus, the adaptive branch of the immune system can kill virally infected cells and generate protective immune memory, which is the basis of vaccination strategies. Both T cell and B cell responses are important in controlling viruses and the development of immunity. However, the COVID-19 pandemic is revealing widely varying immune responses and diverse clinical outcomes with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, raising questions about how antiviral responses are orchestrated, factors that influence the longevity of immunological memory, and approaches that mediate robust protection from viral infections [7,8]. On the other hand, it is the innate immune response that appears to determine the course of infection in particular, the frequency and expression of CD16 by neutrophils, monocytes, NK cells, and immature granulocytes, severe COVID-19 individuals had significantly lower circulating CD16+ NK cells in compared with healthy individuals, but the degree to which the innate immune response confers protection or induces pathogenesis through a dysregulated immune response remains unclear [9,10].
Moreover, variables pertaining to the pathogen-host interaction could be decisive to define the course of the disease in infected patients. Applying viral-track to bronchoalveolar-lavage samples from severe and mild COVID-19 patients reveals a dramatic impact of the virus on the immune system of severe patients compared to mild cases. In this paper, using a systems immunology approach, variables pertaining to the pathogen-host interaction were taking into account, including the following: dynamic of viral shedding, the role of initial viral load, the dynamic of the viral burden, and its relation with the occurrence of cytokine storm, and the relation of these with the ongoing of the COVID-19 from asymptomatic towards severe or critically affected [11,12]. The present study hypothesizes over the conditions that characterize the fraction of the population which get infected by SARS-CoV2, remains asymptomatic or mildly infected, while another fraction being severely symptomatic, regarding SARS-CoV-2 infection in humans. Also, shed some light on the dynamics of the cytokine storm syndrome associated with COVID-19 [13].
Theoretical Considerations
Within-host dynamics (WHD) models differ from most studies in the domain of theoretical immunology in that the higher level (demographic events, epidemiology) is explicitly considered, whereas theoretical immunology typically focuses strictly on within-host processes [14]. Thus, an important aspect of theoretical immunological models is that hosts do not die, whereas, in contrast, in WHD models it is often the explicit aim to assess the effect of a parasite on its host’s mortality (which is one of the standard definitions of virulence). Within-host dynamics models are strongly linked to evolutionary epidemiology.
To develop a versatile strategy to study the complex issues of the within-host SARS- CoV-2 kinetic model, a system dynamic approach is built through the identification and description of various parameters and variables that express system behavior (Table 1) [15,16]. The present model consists of two components, the first one corresponds to the interaction between two populations, the immune cells and the virus SARS-CoV-2; the second part corresponds to cytokine release as a response to the antigenic stimulus induced by the interaction between the immune system and SARS-CoV-2 virus.
Two species interactions
This is a two populations model where and represent, respectively, the immune cells and virus populations at time t= 14 days, which corresponds to the Incubation period (from infection to symptoms). Following Lauer et. al., the median incubation period is estimated to be 5.1 days (95% CI, 4.5 to 5.8 days), and 97.5% of those who develop symptoms will do so within 11.5 days (CI, 8.2 to 15.6 days) of infection [17]. These estimates imply that, under conservative assumptions, 101 out of every 10,000 cases (99th percentile, 482) will develop symptoms after 14 days (336 hours) of active monitoring or quarantine.
In constructing a model of the interaction of the two populations, the following assumptions are made.
In the absence of the immune response, which could be innate immune response (IIR) or adaptative immune response, the viral load grows at a rate proportional to the current population; thus =α α > = and IIR =0.
Table 1:System dynamic model parameters.
| Component (symbol) | Description (units) | Value |
|---|---|---|
| State variables | ||
| Viral burden (v) | Log10 copies/ml | Initial value=1 |
| Immune cells (i) | Log10 cells/ml | Initial value=1 |
| Innate immune response (IIR) | Real value | (0,1) |
| Cytokine’s concentration (c) | pg/ml | Initial value=1 |
| Parameters | ||
| Initial viral load (α) | copies/ml | 0≤α≤10 |
| Interaction rate (β) | Contacts between immune cells and virus/ml*hour | 0.96 |
| Immune cells death rate () | cells/ml* hour | 0.11 |
| Maximum production rate (M) | % Cytokine concentration | 0≤M≤1 |
| Cytokine decline rate (δ) | pg/ml*hour | 0.1 |
In the absence of the virus load the immune cells dies out; thus
The number of encounters between the immune cells and virus is proportional to the product of their populations. Each of such encounters tends to promote the growth of the immune cells, by clonal expansion, and to inhibit the growth of the viral burden. Thus, the growth rate of the immune cells is increased by a term of the formβ vi ,while the growth rate of the viral burden is decreased by a term−β vi , and β is a positive constant.
As a consequence of these assumptions, it is possible to obtain the set of equations described as follows, starting with the population of viral particles or viral burden (v) (1)
Equation (1) represent the variation in time of the virus population within the host as function ofα v , being α the initial viral load. The last part of the equation (1) means the viral decay by the action of the Innate Immune Response; IIR This is crucial for the host defense in the first phase of a new infection, and plays a central role in the response against SARS-CoV-2, which can explain why most of the infected people will never develop COVID-19 or present mild symptoms (80.9%); Thus, the innate immunity is generally able to eliminate pathogens efficiently [9,18,19].
Moreover, the host innate immune system detects viral infections by using pattern recognition receptors (PRRs) to identify pathogen‐associated molecular patterns (PAMPs). At present, the known PRRs mainly include toll-like receptor (TLR), RIG-I-like receptor (RLR), NOD-like receptor (NLR), C‐type lectin-like receptors (CLmin), and free-molecule receptors in the cytoplasm, such as cGAS, IFI16, STING, DAI, and soon. In the present study, the innate immune response against SARS-CoV-2 is defined assuming that an optimal or adequate innate immune response eliminates in its entirety (100%) the pathogens with which it interacts, and a suboptimal IIR is characterized by fail to control the virus in some degree. Under these conditions, IIR is defined as a characteristic function from [0,1] to [0,1], as follows: [20]
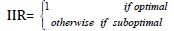
Now, for suboptimal Innate immune response ( IIR<1), three immunotypes (quartiles) are defined inspired by clinical reports for COVID-19 [6],

Meaning respectively, up to 75% efficiency in the clearing of the pathogen, up to 50% and up to 25%. On the other hand, the initial Viral load (α) and the viral burden (v) , are expressed, respectively, in log10 copies/ml*hour, in short (copies/ml*h), and log10 copies/ml, in short (copies/ ml) and following To KK-W et. al., their values are chosen in the real interval (1-10) [21]. The interaction rate (β = 0.96) between the virus particles and the immune effector cells ( vi ) is obtained from Hernandez-Vargas & Velasco-Hernandez, who derived it by fitting numerical estimations comparing the performance of their mathematical model with the data obtained from nine patients with COVID-19 [22].
The innate immune system inhibits virus replication, promotes virus clearance, induces tissue repair, and triggers a prolonged adaptive immune response against the viruses, the latter is represented in the present model by the population of immune effector cells (i) .
Equation (2) represent the dynamic of immune effector cells as a function of the interaction rate β between the virus particles with the immune effector cells ( vi ), and the death rate (γ) of immune effector cells. Regarding the immune effector cells (i) , their values are expressed as log10 of the number of effector cells /ml. The death rate of immune effector cells (γ = 0.11) is taken from Macallan D et.al., who measured, using In vivo labeling with 2H2-Glucose and cell sorting methods, the proliferation and disappearance of immune effector cells in healthy human volunteers [23].
Cytokine release
Cytokines play a central role in limiting the viral spreading within the host during the early phases of the disease. The dynamic of cytokines (C) release is represented by equation (3).
The dynamic behavior of cytokine release follows a mass action law, where the stimulus is the product between the immune cells and the viral burden ( vi ). M, following, Waito et.al., is defined as the maximum cytokine production rate expressed as a percentage [24]. On the other hand, in the absence of these stimulated producers, the number of cytokines will rapidly decline at a rate δ , assuming that viral decay exerts an appreciable influence on cytokine decline [25]. Finally, to record the dynamic of cytokine release the Log10 of average cytokine concentration is used.
Results
The dynamic simulation of the differential equation model (1), (2), and (3), under the conditions and parameters specified in table 1, which seeks to represent the interactions between SARS-CoV-2 and the immune cells, gives rise to a series of interesting results.
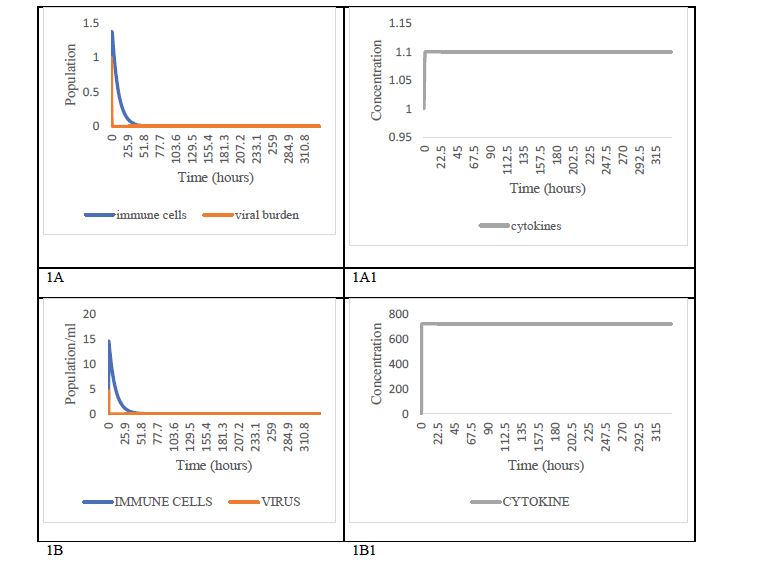
The first scenario corresponds to patients with an optimal innate immune response (IIR=1), conditions under what the infection does not progress for any viral load equal to or less than 1 (Figure 1A), and cytokine levels remain practically constant after a very slight increase as can be seen in Figure 1A1. For greater than 1 (2≤α≤10 copies/ml), it is also found that the infection is rapidly controlled (Figure 1B), but the simulation suggests a notable increment in cytokines levels in these patients (Figure 1B1).
Figure 1: Dynamic scenarios under optimal innate immune response condition. Scenario under conditions of optimal innate immune response (IIR = 1) with low viral load (α≤1) in box 1A. The effect of these conditions on cytokine dynamics is shown in box 1A1. On the other hand, in box 1B, the scenario is presented under conditions of optimal immune response (IIR=1), but high viral load (α〉1). Its impact on cytokine dynamics corresponds to box 1B1. We suggest that scenarios 1A and 1A1 represent fully asymptomatic patients, and scenarios 1B, 1B1 mild symptomatic patients.
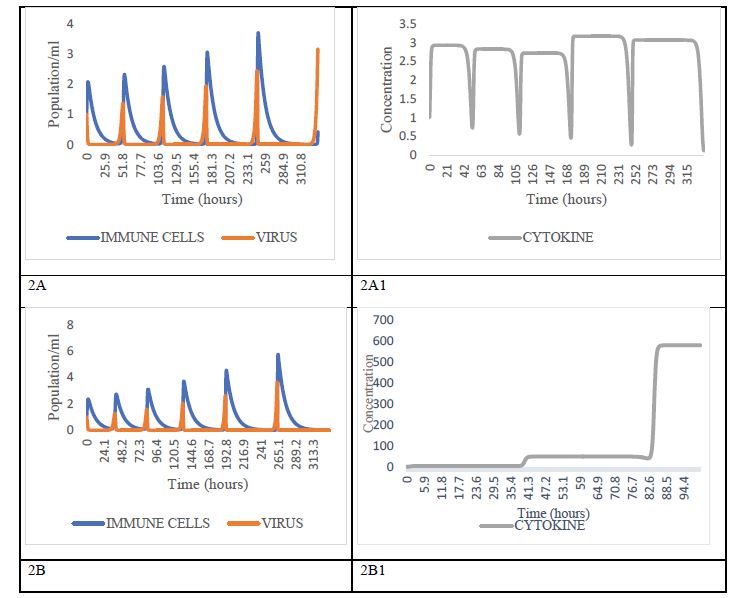
The second scenario corresponds to cases of innate deficient or suboptimal immune response, i.e., 0.1≤IIR<1As can be observed in figure 2 [26]. With IIR= 0.75 and the initial viral load, a is more than 1 copy/ml, there is a phenomenon of viral shedding following an increasing oscillatory behavior, similar to the behavior of a predator-prey system, both in the frequency and level of viral load (copies/ml) (Figure 2A). the phenomenon that is amplified when the innate immune response is more deficient (IIR≤0.5) and the initial viral load is bigger (Figure 2B). It is interesting to note that, with lower viremia, for the present case, 0.1≤α≤0.5 copies/ ml, virus shedding events occur only when IIR= 0.1. The above explains clinical observation related to positive RTPCR results in patients who have recovered from COVID-19 [27,28]. Following Li and collaborators, the prolonged shedding of virus from recovered patients is not an isolated phenomenon, but an integral component of the interaction dynamics between SARS-CoV-2 and the immune cells [29].
Also, numerical results seem to indicate that, with low viral loads, even if the individual does not have an optimal IIR, it is possible not to develop COVID-19 disease, a fact that reinforces the benefits of the social distancing policies and hygiene measures associated with the use of face masks, among others, that have been adopted throughout the world.
Figure 2:Dynamic behaviors with high viral load and suboptimal innate immune response condition. The worst scenarios: high viral load with deficient innate immune response (IIR≤0.75), corresponding probably with severe symptomatic individuals. 2A, 2B the coupled dynamics of immune cells and virus burden resembling recurrence of positive of SARS-CoV-2 RNA in COVID-19 patients. Boxes 2A1 and 2B1 represent the cascade of cytokines in patients with deficient innate immune response, IIR=0.75 with 1 ≤ α ≤ 10 In box 2A1 and (IIR≤0.25) with 1 ≤ α ≤ 10 in box 2B1. Immune of these patients surely dies, because the pro-inflammatory cytokines are detrimental. Box 2B1 shows the well-known cytokine’s storm.
On the other hand, when the dynamics of cytokine levels are taken into consideration, the first thing that should be emphasized is that, under infection conditions characterized by the occurrence of oscillatory virus shedding, the concentration of cytokines has an oscillatory (Figure 2A1), and when IIR=0.25 it is observed a continuous increasing behavior of the cytokine’s levels leading to the well-known clinical phenomenon of cytokine storm (Figure 2B1). Furthermore, the model suggests that the cytokine storm syndrome is also a result of positive feedback generated by the specific combinations of initial viral load numbers and the current innate immune system status of the patients [30].
In the literature on the clinical course of severe and critical COVID-19 patients, the deregulated production of the pro-inflammatory cytokines contributes to inflammation, tissue damages, and in acute COVID-19 patients, deposition of fibrin and platelet microthrombi in the lung vasculature, which contributes to progressive respiratory dysfunction and eventually to death [31]. It’s the fine-tuning between the molecules restricting the growth of pathogens and proinflammatory molecules which maintain the balance and helps in running the host system effectively, which seems to depend on the initial conditions of viral load and innate immune status [32-34].
The present work sheds some light regarding SARSCoV- 2 infection in humans, but their role in the transmission deserves further studies to examine the clinical course of the infection, viral dynamics, viral loads, and immune status, to estimate their real contribution to the transmission dynamic of SARS-CoV-2. Such studies will enhance the understanding of the pathogenesis of these emerging viruses and will inform policymakers to make scientifically sound recommendations.
Limitations
Some limitations of the study are the following. First, it is assumed that the interaction between viral particles and immune response effecting cells is of a symmetrical nature, when in fact it is mediated by the invasion of the virus into the epithelial cells of the lungs, which generates the stimulus that causes their interaction with the immune cells. On the other hand, it is assumed that the innate immune response acts as a simple characteristic function, which oversimplifies the chain of events related to the non-specific response, but allows the obtaining of a set of interesting results that explain some clinical observations associated with COVI-19, such as the cytokine storm.
Moreover, it is important to point out that the values assigned to those parameters of our models are taken for the reported studies, the simulation results must be understood more as dynamical patterns than precise predictions, like any other mathematical approximation in immunology.
Conclusion
The present study hypothesizes over the conditions that characterize the fraction of the population which get infected by SARS-CoV2; the asymptomatic patients as those with optimal innate immune response, exposed at any virus loads and controlling the viremia in few hours after infection. These could be not infective when maintain contact with other people. The asymptomatic or symptomatic mildly infected patients, as those possessing a low viral burden with shedding episodes, given their suboptimal innate immune response, these patients could be infective to other people. The combination of high viral loads with deficient innate immune response led to acute or severe symptomatic COVID-19 patients, characterized by suffering viral shedding episodes frequently and with an increasing concentration of circulating cytokines. Some of these patients surely die, because the pro-inflammatory cytokines are detrimental, causing the cytokine storm with increasing viral load, tissue damages and pathophysiology.
Traditionally, public health interventions were based on data collected without reference to the mechanisms underlying the patterns being observed, as is the case, for example, in studies of cohorts, or in clinical trials. An increase in the perceived usefulness of getting to understand the mechanisms underlying the patterns observed in public health data has precipitated integration with disciplines such as immunology. To the benefit of public health, much integration has already occurred, systems immunology being an example. Public health researchers can, therefore, adopt emerging integrative paradigms that link populationlevel processes to molecular mechanisms. As a result, those in public health research are turning increasingly to WHD models to help determine how best to reduce transmission rates and hence morbidity.
Meanwhile, the past history of disease, even recent pandemics, when well-documented, can provide the necessary data for the testing of WHD models and consequently their pattern predictive abilities. In the future, such an amalgamation between public health data, disease processes and eco-immunology should provide the basis for better strategies in combatting the spread of infection and the resulting morbidity.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics Statements
No animal studies are presented in this manuscript.
No human studies are presented in this manuscript.
No potentially identifiable human images or data is presented in this study.
Data Availability Statement
All datasets presented in this study are included in the article.
Li Q, Guan X, Wu P (2020) Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med 382: 1199-1207. [ Ref ]
World Health Organization. (2020) Novel Coronavirus (2019‐nCoV) situation report‐2. [ Ref ]
Giordano G, Blanchini F, Bruno R, Colaneri P, Di Filippo A, et al. (2020) Modelling the COVID-19 epidemic and implementation of populationwide interventions in Italy. Nature Medicine 26: 855-860. [ Ref ]
Wadman M, Couzin-Frankel J, Kaiser J, Matacic C (2020) How does coronavirus kill? Clinicians trace a ferocious rampage through the body, from brain to toes. Science. [ Ref ]
Netea M, Quintin J, van der Meer J (2011) Trained immunity: a memory for innate host defense. Cell Host Microbe 9: 355-361. [ Ref ]
Chen N (2020) Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. The Lancet 395: 507-513. [ Ref ]
Hope JL, Bradley LM (2020) Lessons in antiviral immunity. Science 371: 464-465. [ Ref ]
Mathew D (2020) Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. [ Ref ]
Kuri-Cervantes L (2020) Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci Immunol 5: eabd7114. [ Ref ]
Mckichnie JL, Blish CA (2020) The innate immune system: Fighting on the front line or fanning the flames of COVID-19. Cell Host & Microbe 27: 863-869. [ Ref ]
Handel A (2020) A software package for immunologists to learn simulation modeling. BMC immunology 21: 1. [ Ref ]
Li G, Fan Y, Lai Y (2020) Coronavirus infections and immune responses. J Med Virol 92: 424-432. [ Ref ]
Hu B, Huang S, Yin L (2021) The cytokine storm and COVID‐19. J Med Virol 93: 250-256. [ Ref ]
Alizon S, van Baalen M (2008) Acute or Chronic? Within-Host Models with Immune Dynamics, Infection Outcome, and Parasite Evolution. The American Naturalist 172: E244-E256. [ Ref ]
Bala B, Arshad M, Noh K (2017) System Dynamics Modelling and Simulation. Springer. [ Ref ]
Ellner S, Gluckenheimer J (2006) Dynamic Models in Biology. Princeton University Press, USA. [ Ref ]
Lauer S, Kyra H, Grantz B, Qifang B (2020) The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann Intern Med 172: 577-582. [ Ref ]
Akira S, Uematsu S, Takeuchi O (2008) Pathogen recognition and innate immunity. Cell 124: 783-801. [ Ref ]
Frieman M, Mark Heiseb M, Bari R (2008) SARS coronavirus and innate immunity. Virus Research 133: 101-112. [ Ref ]
Netea MG, Josten LAB (2018) Trained Immunity and Local Innate Immune Memory in the Lung. Cell. 175: 1463-1465. [ Ref ]
To KK (2020) Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARSCoV- 2: an observational cohort study. Lancet Infect Dis. 20: 565-574. [ Ref ]
Hernandez-Vargas, Velasco-Hernandez (2020) In-host Mathematical Modelling of COVID-19 in Humans. Annual Reviews in Control 50: 448- 456. [ Ref ]
Macallan D (2004) Rapid turnover of effector memory CD4 T cells in healthy humans. J Exp Med 200: 255-260. [ Ref ]
Waito M, Walsh SR, Rasiuk A, Bridle BW, Willms A (2016) A Mathematical Model of Cytokine Dynamics During a Cytokine Storm. Mathematical and Computational Approaches in Advancing Modern Science and Engineering. Springer International Publishing, Switzerland. [ Ref ]
McDade T, Georgiev A, Kuzawa C (2016) Trade-offs between acquired and innate immune defenses in humans. Evolution, Medicine, and Public Health 2016: 1-16. [ Ref ]
Nelemans T, Kikkert M (2019) Viral innate immune evasion and the pathogenesis of emerging RNA virus infections. Viruses 11: 961. [ Ref ]
Lan L (2020) Positive RT-PCR tests results in patients recovered from COVID-19. JAMA 323: 1502-1503. [ Ref ]
Yang J (2020) Persistent viral RNA positivity during the recovery period of a patient with SARS-CoV-2 infection. J Med Virol 92:1681-1683. [ Ref ]
Li N (2020) Prolonged SARS-CoV-19 RNA shedding: not a rare phenomenon. J Med Virol 92: 2286-2287. [ Ref ]
Yang Liu Y, Yan LM, Wan L, Xiang TX, Le A, et al. (2020) Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis 20: 656-657. [ Ref ]
More J, June C (2020) Cytokine release syndrome in severe COVID-19. Science 368: 473-474. [ Ref ]
Al-Tawfiq J (2020) Asymptomatic corona virus infection: MERSCoVandSARS- CoV-2. Travel Medicine and Infectious Disease 35: 101608. [ Ref ]
Rothe C, Schunk M, Sothmann P (2020) Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. 2020. N Engl J Med 382: 970-971. [ Ref ]
Liu J, Li S, Liu J (2020) Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 55: 102763. [ Ref ]