Journal Name: Journal of Multidisciplinary Research and Reviews
Article Type: Research
Received date: 11 August, 2020
Accepted date: 23 September, 2020
Published date: 30 September, 2020
Citation: Rafique S, Nasrullah N (2020) Characterization of Plasma Membrane Proteins from Maize Roots (Zea mays L.) under Multiple Abiotic Stresses using LC-MS/MS Technique. J Multidis Res Rev Vol: 2, Issu: 2 (11-23).
Copyright: © 2020 Rafique S et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Maize inbred plants showed tolerance when exposed to various abiotic stresses simultaneously and in combination (drought x low-N and waterlogging x low-N stress). The stressed maize plants had higher photosynthetic efficiency, shows increase in plant height, leaf area, and they also maintained high leaf relative water content in drought x low-N stress and not much reduction in morphological parameters under combined stresses. Therefore, to understand the mechanism regulating the tolerance to multiple stresses, we analyzed maize roots plasma membranes proteins of stressed plants by using LC-MS/MS techniques, the large number of proteins (295) were identified which were mainly trans- membrane proteins, low abundance proteins, and root specific proteins. Among various proteins characterized, only four proteins were selected like high-affinity Nitrate transporter, NR enzyme, PEP carboxylase, Glutamine synthetase proteins and their induction were validated by qRT-PCR approach in control and stressed plants. The qRT-PCR results exhibits that in control and stressed plants the gene of all four proteins were equally expressed. We concluded the highaffinity nitrate transporter proteins in stressed plants might represent the executive part of the protective response that plays a significant role in particular low-N stress tolerance along with NR enzyme, PEP carboxylase and glutamine synthetase. While, presence of other major proteins like kinases, stress-responsive TFs, calmodulin, aquaporins, stress-related proteins, and many more proteins and their interaction with nitrate transporter proteins and their role can be validated only after comparisons between control and treated samples based on the same peptide mass-to-charge ratios (m/z) that were acquired under the same general conditions during LC-MS/MS experiments.
Keywords
Multiple abiotic Stresses, Zea mays, LCMS/MS, Roots plasma membrane, Nrt2.1, Photosynthetic efficiency.
Abstract
Maize inbred plants showed tolerance when exposed to various abiotic stresses simultaneously and in combination (drought x low-N and waterlogging x low-N stress). The stressed maize plants had higher photosynthetic efficiency, shows increase in plant height, leaf area, and they also maintained high leaf relative water content in drought x low-N stress and not much reduction in morphological parameters under combined stresses. Therefore, to understand the mechanism regulating the tolerance to multiple stresses, we analyzed maize roots plasma membranes proteins of stressed plants by using LC-MS/MS techniques, the large number of proteins (295) were identified which were mainly trans- membrane proteins, low abundance proteins, and root specific proteins. Among various proteins characterized, only four proteins were selected like high-affinity Nitrate transporter, NR enzyme, PEP carboxylase, Glutamine synthetase proteins and their induction were validated by qRT-PCR approach in control and stressed plants. The qRT-PCR results exhibits that in control and stressed plants the gene of all four proteins were equally expressed. We concluded the highaffinity nitrate transporter proteins in stressed plants might represent the executive part of the protective response that plays a significant role in particular low-N stress tolerance along with NR enzyme, PEP carboxylase and glutamine synthetase. While, presence of other major proteins like kinases, stress-responsive TFs, calmodulin, aquaporins, stress-related proteins, and many more proteins and their interaction with nitrate transporter proteins and their role can be validated only after comparisons between control and treated samples based on the same peptide mass-to-charge ratios (m/z) that were acquired under the same general conditions during LC-MS/MS experiments.
Keywords
Multiple abiotic Stresses, Zea mays, LCMS/MS, Roots plasma membrane, Nrt2.1, Photosynthetic efficiency.
Introduction
Agricultural system is widely affected with various environmental factors. Recent climate prediction models indicates that rise in temperature, frequent occurrence of drought, flooding and heat waves are major constrains which are causing higher agricultural production loses [1,2]. Hence, the understanding of plant responses to various abiotic stresses is utmost crucial. Plants are sessile organism, their survival depends on coping with the environmental challenges. The abiotic stresses either singly or in combination cause significant damage to crop plants. The combination of two different stresses might have synergistic effect that may enhance tolerance of the plant or or different abiotic stresses antagonize and exaggerate the effects of each other [3]. Though the plant response to different stresses is highly complex and involves changes at the transcriptome, proteome and physiological levels [4]. Perhaps, plasma membranes are primary sites that received signals for the cellular level changes in protein expression and these signals transfer to the cell. Hence, plasma membranes are structural barrier through which exchange of substances and information are communicated to the extracellular environment of the cell [5]. Most of the signal and transporter proteins are embedded in the plasma membrane these integral membrane proteins contains trans-membrane domains (hydrophobic nature), they cannot be solubilized in 2DE buffers [6]. Therefore, to identify diverse arrays and a wide range of proteins, particularly basic proteins, low-abundance, small proteins and hydrophobic proteins thus LCMS/MS-based-based proteomics is a parallel method with higher efficiency [7]. Also it considered as highly sensitive, accurate method for identifying the integral membrane proteins. Maize is a staple food crops in the tropical climate, largely grown in marginal areas of rain fed system. during summer rainy season it has to face both drought and waterlogging stresses due to uneven distribution patterns of monsoon rains in the region [8]. However at the beginning of plant growth, occurrences of these two stresses may limit the photosynthetic ability of leaves and biomass gain at the vegetative stage. Further nitrogen availability is low under both these stresses, water deficit affects the N-uptake while, in waterlogging leaching and de-nitrification causes the depletion of nitrogen [9]. Since the nitrogen is the major nutrient that influences the growth of plants and roots are the main organs through which mineral nutrients are taken up. Besides, roots are the first organ that perceives abiotic stress signals, weather due to anoxia (waterlogging), cell wall remodeling under water deficit or nutrient deficiency (N or P deficiency) henceforth they are essential for plant growth, survival and fitness. it has been reported that the root is a useful tissue for proteomic research. Therefore, roots of the stressed maize plants was selected for physiological studies and the role of root plasma membrane proteins was investigated using LCMS/MS technique under various combined abiotic stress conditions.
Figure 1a,b:Maize plants shows no phenotypic differentiation between control (a) and stressed (b) plants under waterlogging x low-N stress (5 days after stress). The stress symptoms are not visible in treated plants like leaf wilting, chlorosis/necrosis, lodging and white tips on the surface rooting.
Materials and Methods
Plant materials and stress conditions
Maize seeds were obtained from the International Maize and Wheat Improvement Center (Spanish acronym; CIMMYT®). [50-VL1018393; 51-VL0512387; 52-VL0512388; 53-VL1012838; 55-VL1018413; 56- VL0512393; 57-VL1018418; 58-VL1018419; 59- VL1018513; 60-VL1018514]. This identified inbred seeds having distinct difference in terms of tolerance/ susceptibility to single stress, like waterlogging, drought and low-N. Maize seeds were sown in earthen pots (10 cm diameter) filled with sandy loam soil. The pots were kept in a naturally lit greenhouse, with air temperature 25°-30° C and relative humidity 55-65%. Ten plants (one inbred line) were chosen with six replications per plants, each pot contains 2 plants per pot after seedling emergence. Fourteen pots for control and 40 for treatment, the nutrient applied in pots was calculated on the basis per kg soil, a full dose of phosphorous potash and zinc was mixed in the soil before sowing without any organic fertilizer. (As per agronomic recommendation is N 120 kg/ha (urea) required by maize plants) However, for Low-N (LN) treatment, 25% N was used only once (195mg N/pot), unlike in control pots normal N rates (780mg N/ pot) was given in split doses. The waterlogging stress was given 30 days after sowing, (Figure 1a, b) for up to 7 days. After completion of the stress, water was drained out from the pots by opening the holes at the bottom. Subsequently, the drought stress was given 40 days after sowing, (without recovery period) by withholding the water for a 10 days (Figure 2a, b). During the stress period soil moisture content of the pots was measured on the 7th day of stress from control and treatment pots (Figure S1 supplementary).
Figure 2a,b:Maize plants under drought x low-N stress control (a) and stressed (b) both the phenotypes (control and stressed) are similar.
Parameters measured under various stresses and Statistical analysis
Growth and morphological observation was recorded like, leaf area, plant height, leaf number, fresh and dry weights of shoots and roots, total fresh and total dry weights of seedlings. Physiological parameters like, Net photosynthesis rate (PN), stomatal conductance (gs), internal CO2 concentration (Ci), transpiration rate (E) was measured using LI-6400 (LI-COR Lincoln NE) portable closed gas exchange system. Three plants of each control and treated plants were chosen, fully expanded leaf blades were enclosed in the assimilation chamber for measuring the photosynthetic rate between 8:30 am to 10:00 am for this Same leaf was plugged for chlorophyll content determination and leaf relative water content (RWC). For all the measured parameters each pot represented one replication. A minimum of three pots were sampled for all observation, the average of three replicates were analyzed using descriptive statistics and paired T-test for each trait and data was expressed on a per plant basis. To verify the significance of the variations of all the parameters, One-way analysis of variance (ANOVA) followed by the post hoc Tukey test (p < 0.01) was used.
Extraction of the membrane proteins
Fresh roots samples was collected in three biological replicates and pooled. They were immediately frozen in liquid Nitrogen and stored at -80 for LCMS/MS experiment and qRT-PCR studies. However, the 3-different roots was collected and stored from control plants for qRT-PCR experiment.
Homogenate preparation and separation of membrane proteins
All extraction procedures were carried out on ice at 4°C. Fresh roots were weighed (5 g) in triplicate (biological) and the tissue was first grounded in liquid N2 then in 10 ml of cold extraction buffer (250 m Sucrose, 1 mM EDTA, 10 mM Tris HCl buffer, pH 7.2 and protease inhibitor) (Sigma P9599).Then homogenate was transfer to centrifuge tube and sonicated using two 10 second pulses (30 seconds in between pulses) using a probe sonication (Bath sonication, 30 KHz frequency), samples kept in ice bath, to minimize the sample-air interface foaming. The intact cell, nuclei and cell debris was removed by centrifugation of the homogenate at 15000 x g for 15 minutes at 4°C (the step was repeated) and the pellet was discarded. Again the supernatant was centrifuge at 100,000 x g for 1 hour at 4°C. The obtained supernatant contains the soluble proteins that were discarded. The pellet was washed by homogenization buffer and re-centrifuge at 100,000 x g, 4°C, for 1 hour. The supernatant was discard and the remaining pellet contains all of the cell’s membrane fraction was kept.
Phenol/Ammonium Acetate-Methanol Precipitation of membrane proteins
Membrane pellet was suspend in 0.5 ml of extraction buffer (0.7 M sucrose, 0.5 M Tris, 30 mM HCl, 50 mM EDTA, 0.1 M KCl , 2% (v/v) 2-mercaptoethanol and 2 mM PMSF) (Sigma-Aldrich) and homogenize. Incubated for 10 min at 4°C and then an equal volume of Tris-saturated (7.5 pH) phenol was added. Centrifuged to separate the phases, the phenol phase was recovered and re-extract with an equal volume of extraction buffer. Proteins was precipitated from the phenol phase by adding of 5 volumes of 0.1 M ammonium acetate in methanol and incubated at -20°C for overnight. The precipitate was washed 3-times with ammonium acetate in methanol and one time with acetone, the pellet was air dried and solubilize in rehydration buffer by incubating for at least 1 hour at room temperature, with occasional vortex and then centrifuge at 100,000 x g, 4°C, for 1 hour. The supernatant was removed and save. The sample protein concentrations were quantified by Bradford’s method. Bradford, using bovine serum albumin (Fischer Scientific) as a standard [10].
Trypsin digestion in solution samples and data analysis in LC MS/MS
The goal of this study was to comprehensive identification of integral membrane proteins. 100μg of roots protein sample was taken for digestion; the volume was made up to 100μl with 50mM NH4HCO3. The sample was treated with 100mM DTT at 95°C for 1 hour, followed by 250mM Iodoacidamid (IA) at room temperature in the dark for 45 min. The sample was then digested with trypsin and incubated overnight at 37°C. The sample was vacuum dried and dissolved in 10μl of 0.1% formic acid in water. After centrifugation at 10000 x gs, the supernatant was collected into the separate tube. 1μl injection volume was used on C18 nUPLC column for separation of peptides, and then followed by analysis on the Water Synapt G2 Q-TOF instrument for MS and MSMS. The raw data was processed by MassLynx 4.1 WATERS. The individual peptides MS/MS spectra were matched to the database sequence for proteins identification on PLGS software, WATERS. For Protein identification Database used UNIPROT, Mass Tolerance; 50ppm and Peptide mass tolerance; 100ppm for the search to proceed. Specific modification; Carbamidomethyl and variable modification; Oxidation (M). Result based on the Scores of the matching protein masses and probable peptides was given as output.
Quantification of gene expression by Semiquantitative RT- PCR
Total RNA was extracted from 250 mg of frozen roots tissue stored at −80° C of treated (after combined stress treatment) and control plants, using the TRIzol method (as described by the manufacturer) An aliquot of total RNA was treated with RQ1 RNase-free DNAse (Promega), to avoid genomic contamination and 1μl of total RNA was quantified by spectrophotometer using a Nanodrop 1000 (Thermo Scientific, Nanodrop Products). First cDNA was synthesized from 100 ng of total RNA and mixed with 1 μl of Oligo dT (10 μM). The reaction was incubated 5 min at 70°C, qRT-PCR was performed with gene-specific primers corresponding to the genes encoding the identified proteins (Table S1 supplementary). The primers were designed to generate PCR products of 500-1000bp. MEP and LUG used as reference gene for normalization of internal cDNA input.
Results and Discussion
Effects of various stresses on maize plants
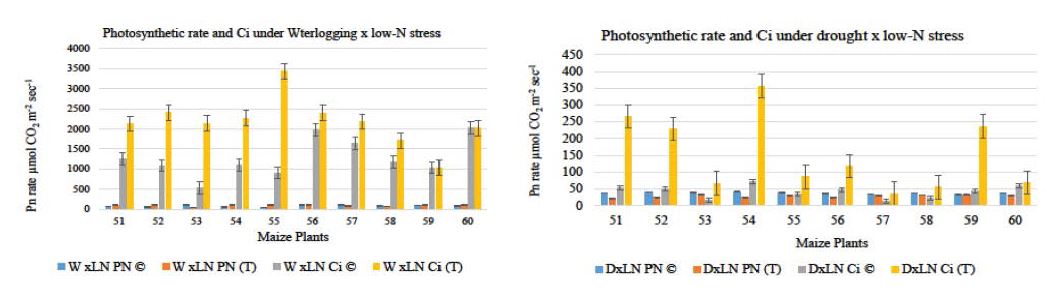
This studies was carried out with the purpose to understand the effects of various stresses applied simultaneously on maize inbred plants at vegetative stage and to reveal the process of tolerance by proteomic approach, specifically the low-N response. The phenotypic observations of treated plants under waterlogging x low-N stress shows no lodging, wilting, leaf necrosis, and surface rooting, instead early brace root development was observed. Subsequently in drought x low-N stress symptoms includes (Fig 1a,b) leaves drooping, yellowing, wilting and premature leaf there was no such phenotype was visible in treated plants (Fig 2a, b) Photosynthesis is among the primary process to be affected under stress condition. The decline in photosynthesis under multiple stress conditions may be due to oxidative stresses, the multiple stresses affect leaf photosynthetic machinery [11,12]. But in our studies we have found that under waterlogging x low-N stress, all treated plants shows higher photosynthetic efficiency (13.77%) relative to control plants. Whereas, in drought x low-N stress the decline in photosynthetic rate was much more compared to waterlogging x low-N stress. Although this decline in photosynthesis was not below the range 40-50μmol CO2 sec-2mol-2 under drought Zaltev and Lidon indicated the sustenance of photosynthetic mechanism by plants under water deficit stress shows the drought tolerance capability [13]. Hence our results indicated that overall photosynthesis sustained in treated plants under multiple stresses (Figure 3a, b). Moreover, in our experiments plants were grown under low-N stress yet maintain photosynthesis. Maize plants shows the strong correlation of photosynthetic rate and leaf N to AEI (assimilation efficiency index). [14]. Also the control and treated plants have shown non-significant difference in their mean values for various growth traits, morphological, and physiological traits (Table 1). Although plants were subjected to multiple stresses yet they maintained high assimilation rate. Therefore, it appears their might be some changes during the stress or some signals were regulated to overcome the stressful conditions. Therefore to determine whether the observed rates of photosynthesis described above correlated with changes in proteins under stress conditions. The complete protein profile of treated maize roots was analyzed in detail by LCMS/MS method (in solution).
Figure 3:Photosynthetic rate of control and stressed plants under waterlogging x low-N drought x low-N stresses. Mean values of Pn rate and internal CO2 concentration (Ci) are significant at p<0.05.
Effects of various stresses on maize plants
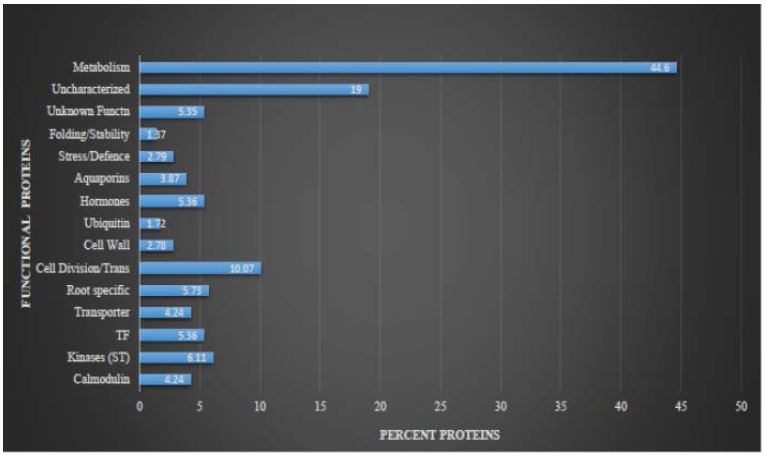
The aim of the study was to understand the response of maize plants under multiple stress conditions. Maize plants subjected to combined stresses (waterlogging x low-N and drought x low-N) and the proteins were extracted from roots and analyzed by LC-MS/MS technique (in solution). In the root tissue of maize, approximately 295 proteins were identified shown in Table 2. Most of the proteins were related to diverse biological functions and has been categorized according to Bevan et al. [15]. The protein percentage was calculated by dividing the type and number of functional proteins from total no proteins present. Proteins related to nitrogen, and carbon metabolism were maximum in number (44.6%). However, some metabolism related proteins were associated with plasma membranes like, Enolase, G-3P dehydrogenase, PEP carboxylase, Nitrate Reductase enzyme. But some of the enzymes are soluble proteins, Alexandersson et al. consider them as contaminants of the plasma membrane preparation [16]. The second maximum were uncharacterized proteins (19%), calmodulin, kinases (Signal transduction), transcription factors (TF), transporter proteins, root specific proteins, cell division, translation and cell wall synthesis proteins, stress related proteins. While others were hormones, ubiquitin related proteins shown in figure 4. The concentration or abundance molecules in the sample can be detected by mass spectrometry. In our experiment, the maximum percentage coverage 95.41%, 94.58% and 75.38% is shown by NRT2.1 protein (Accession No’s: Q53CL7, Q0VH26, and Q0VH25). The highest peak of the chromatogram (Figure S2 supplementary), also indicate the high expression of NRT2.1 proteins in the roots samples. Another maximum percent coverage was membrane bound Nitrate Reductase proteins (Accession No. Q4U5G4, 95.35%).
Table 1:The various traits measured under combined abiotic stresses in control and treated maize plants. The difference between control and treated was significant and low. Mean ± SE (m) and C.D values.
| Trait | Treatment | Waterlogging x Low-N stress | Drought x Low-N stress | ||||
|---|---|---|---|---|---|---|---|
| Mean | C.D | SE(m) | Mean | C.D | SE(m) | ||
| Photosynthesis rate (μmol CO2 m-2 S-1 | Control Treated | 80.733** 93.68 | 24.1 | 8.401 | 38.233* 28.623 | 6.35 | 2.21 |
| Conductance (gs) | Control Treated | 0.087** 0.101 | 0.023 | 0.008 | 0.264** 0.142 | 0.107 | 0.037 |
| Internal CO2 (Ci) | Control Treated | 1819.47* 1902.20 | 1785.61 | 622.433 | 57.267** 136.315 | 117.098 | 40.818 |
| Transpiration rate E (mol) | Control Treated | 2.428** 2.722 | 0.943 | 0.329 | 4.742** 2.83 | N/A | 0.538 |
| Plant height (cm) | Control Treated | 33.9** 31.5 | N/A | 3.251 | 29.167** 28.933 | N/A | 3.234 |
| Leaf area (cm-2) | Control Treated | 1746.54** 1146.85 | N/A | 334.343 | 1344.42* 1236.78 | N/A | 321.882 |
| Leaf No. (per plant) | Control Treated | 7.8* 7.0 | N/A | 0.699 | 6.633* 6.667 | N/A | 0.587 |
| Fresh shoot weight (g) | Control Treatment | 72.42** 45.215 | N/A | 12.9 | |||
| Dry shoot weight (g) | Control Treatment | 14.718* 9.857 | N/A | 3.094 | |||
| Fresh root weight (mg) | Control Treated | 10.12** 4.243 | 7.061 | 2.461 | |||
| Dry root weight (mg) | Control Treated | 2.43** 1.042 | N/A | 0.613 | |||
| Total fresh weight (g/plant) | Control Treated | 85.643* 51.215 | N/A | 17.887 | |||
| Total dry weight (g/plant) | Control Treated | 17.154** 11.442 | N/A | 3.481 | |||
| **significant difference for p < 0/ 0.001 *significant difference for p < 0.05/ 0.01 | |||||||
Possible role of NRT2.1 proteins, Nitrate Reductase, Phosphoenol pyruvate carboxylase and Glutamine synthetase and their validation using qRT-PCR studies in control and stressed plants under multiple stresses
The maize plants were grown under low-N stress, and we have identified the nitrate transporter protein in treated plants. The mRNA transcript of high affinity nitrate transporter in treated plants was spotted by qRT-PCR studies. Miller et al. also emphasize the regulation of HATS at the range of above 1mM concentrations (Soil low-N), Thus confirms their induction at low NO-3 concentration [17]. Similarly, the induction of NRT2.1 genes at very low levels of NO-3 (10–50 mM) was noted in N. plumbaginifolia and Arabidopsis [18,19]. The nitrate transporter transcripts was also detected in control plants (Fig 5).
The presence of two types HATS was detected in barley the one was iHATS, and other cHATS, at low NO-3 concentration, similar to our results the constitutive HATS (cHATS) in control plants whereas, inducible HATS (iHATS) transcripts in treated plants [20]. Moreover, possible role of NRT.2 proteins might to maintain N homeostasis in various stresses. In similar work the roots of two salt cultivars (salttolerant FL478 and salt-sensitive IR29 rice varieties) had
Table 2:Plasma membrane proteins of maize roots expressed under multiple abiotic stresses and identified by LC-MS/MS technique.
| Protein Name a | Accession No b | MW(Da) d | Peptide e | Theoretical f | %Coverage g | |
|---|---|---|---|---|---|---|
| Calcium Binding Proteins | ||||||
| Calmodulin (fragment) | U5Q018_MAIZE | 12554 | 4.04 | 24 | 15 | 100 |
| Calmodulin (fragment) | U5Q0B5_MAIZE | 11879 | 4.08 | 21 | 13 | 97 |
| Calmodulin (fragment) | U5PZT9_MAIZE | 10511 | 4.16 | 21 | 11 | 100 |
| Calmodulin (fragment) | U5Q0D5_MAIZE | 9305 | 4.07 | 16 | 10 | 100 |
| Calmodulin (fragment) | U5Q0C3_MAIZE | 12291 | 3.83 | 19 | 12 | 100 |
| Calmodulin (fragment) | U5PZT0_MAIZE | 12703 | 4.01 | 20 | 14 | 98 |
| Calmodulin (fragment) | U5PZT0_MAIZE | 12703 | 4.01 | 20 | 14 | 98 |
| Calmodulin (fragment) | U5Q0C8_MAIZE | 10553 | 4.16 | 17 | 11 | 96 |
| Calmodulin (fragment) | U5PZQ0_MAIZE | 10665 | 4.19 | 23 | 11 | 95 |
| Calmodulin (fragment) | U5Q3A9_MAIZE | 12583 | 4.04 | 26 | 16 | 100 |
| Calmodulin-binding protein | Q41796_MAIZE | 14604 | 5.06 | 21 | 8 | 69 |
| Calcium-binding protein | Q43712_MAIZE | 47983 | 4.28 | 46 | 45 | 91 |
| Calcium transporting ATPase | A0A096PNP4_MAIZE | 97157 | 7.59 | 107 | 56 | 87 |
| Calmodulin-binding protein(F) | Q41797_MAIZE | 31814 | 9.42 | 35 | 19 | 87 |
| Calmodulin (Fragment) | U5Q0C8_MAIZE | 10553 | 4.16 | 17 | 11 | 96 |
| Calmodulin (Fragment) | U5PZQ0_MAIZE | 10665 | 4.19 | 23 | 11 | 95 |
| Annexin | Q43864_MAIZE | 35237 | 6.91 | 40 | 33 | 75 |
| Protein Kinase/Signal Transduction | ||||||
| Ca2+dependent protein kinase | Q41789_MAIZE | 50564 | 4.95 | 51 | 51 | 93 |
| Putative Serine/threonine -specific Protein kinase (Fragment) | Q6B7Q8_MAIZE | 18932 | 8.45 | 14 | 13 | 92 |
| Somatic embryogenesis receptor | Q8LPS5_MAIZE | 66939 | 5.33 | 59 | 36 | 90 |
| Adenosine Kinase (Fragment) | Q9XGC6_MAIZE | 36009 | 5.04 | 35 | 23 | 71 |
| CAK1AT Kinase –like Protein | B6TPK0_MAIZE | 52427 | 4.20 | 53 | 31 | 99 |
| Putative receptor protein kinase | Q93XG1_MAIZE | 36134 | 6.44 | 60 | 34 | 99 |
| SNF1-related protein kinase | P17801_MAIZE | 91062 | 6.48 | 88 | 60 | 91 |
| Putative leucine-rich repeat receptor-like protein | Q6RXY2_MAIZE | 18538 | 8.46 | 20 | 12 | 92 |
| Tousled-like kinase1(F) | K7V4X2_MAIZE | 120450 | 5.68 | 95 | 76 | 81 |
| MKK6-putative MAPKK | Q6DUC4_MAIZE | 70985 | 7.33 | 79 | 59 | 81 |
| Diacylglycerol kinase | O49975_MAIZE | 39849 | 5.46 | 42 | 30 | 90 |
| Inositol1,3,4,5,6-pentakis Phosphate 2 kinase | C0PCE8_MAIZE | 77215 | 8.00 | 73 | 52 | 85 |
| Zinc finger protein MAGPIE | A6YH14_MAIZE | 48935 | 7.24 | 47 | 38 | 83 |
| kinase CRINKLY4 | Q9ZWA6_MAIZE | 55791 | 8.29 | 38 | 32 | 70 |
| CDPK-related protein kinase | Q41792_MAIZE | 67356 | 9.23 | 66 | 50 | 83 |
| ATP Sulfurylase | O48888_MAIZE | 53752 | 9.08 | 65 | 50 | 89 |
| BRASSINOSTEROIDE | Q94F62_MAIZE | 68118 | 5.51 | 56 | 37 | 86 |
| INSENSITIVE1-Associated receptor kinase Hexokinases | Q8L5G8_MAIZE | 54782 | 6.03 | 66 | 36 | 96 |
| Transcription Factors | ||||||
| OCS element-binding factor1 | P24068_MAIZE | 16965 | 9.34 | 19 | 13 | 84 |
| GRAS transcription factor | C0PGA9_MAIZE | 63865 | 5.61 | 53 | 40 | 90 |
| Transcription factor MYB31 | Q2A702_MAIZE | 31075 | 8.03 | 38 | 22 | 87 |
| Transcription factor MYB42 | Q2A700_MAIZE | 28187 | 7.86 | 30 | 19 | 86 |
| GRAS transcription factor (F) | A0A060D7Z4_MAIZE | 63716 | 5.37 | 53 | 42 | 93 |
| AP2-EREBP transcription factor | Q945C8_MAIZE | 36866 | 4.93 | 22 | 20 | 85 |
| Transcription factor MYB8 | Q9XHR2_MAIZE | 24223 | 8.84 | 21 | 15 | 87 |
| Putative MYB DNA-binding domain superfamily protein | Q8RXB5_MAIZE | 33493 | 9.45 | 27 | 25 | 93 |
| PHD Transcription factor | B6U670_MAIZE | 24968 | 7.94 | 26 | 17 | 88 |
| WUSCHEL-related homeobox 9 | Q8W0F1_MAIZE | 22806 | 8.27 | 34 | 23 | 91 |
| Cell Division/Translation Proteins | ||||||
| Telomerase reverse transcriptase | Q1EG33_MAIZE | 125900 | 9.65 | 123 | 90 | 85 |
| Cell division protein FtsZ | Q8RMK5_MAIZE | 26621 | 5.96 | 30 | 17 | 71 |
| DNA replication licensing factor MCM3 | Q9SX03_MAIZE | 85155 | 5.89 | 123 | 77 | 87 |
| Eukaryotic translation initiation factor 5A-2 | Q93VP3_MAIZE | 17129 | 5.49 | 23 | 17 | 94 |
| Replication origin activator 4 | Q9SX02_MAIZE | 19925 | 8.48 | 29 | 24 | 97 |
| Translation elongation factor-1 alpha | A6YDJ4_MAIZE | 11013 | 9.43 | 10 | 9 | 81 |
| Eukaryotic translation initiation factor 3 subunit A | Q9XHR2_MAIZE | 111494 | 9.62 | 144 | 84 | 85 |
| Origin recognition complex | Q945C8_MAIZE | 91672 | 7.95 | 104 | 77 | 90 |
| Zinc finger protein NUTCRACKER | Q9FFH3_MAIZE | 51157 | 7.98 | 31 | 30 | 71 |
| Protein Related To Carbon Metabolism | ||||||
| Cytosolic G3-P dehydrogenase | Q43359_MAIZE | 36427 | 6.69 | 39 | 26 | 83 |
| Malate dehydrogenase | Q93XD0_MAIZE | 11883 | 4.85 | 14 | 8 | 97 |
| Enolase | P26301_MAIZE | 48033 | 5.01 | 58 | 34 | 98 |
| Trehalose-6-phosphate | K7V516_MAIZE | 107148 | 6.31 | 109 | 67 | 92 |
| Phosphoenolpyruvate carboxylase | Q9SAZ6_MAIZE | 109360 | 5.68 | 123 | 87 | 93 |
| Hexokinase | Q8L5G8_MAIZE | 54782 | 6.03 | 66 | 36 | 91 |
| Acyl CoA synthetase | B6SYY5_MAIZE | 73940 | 6.78 | 82 | 46 | 81 |
| Glucose-6-phosphate isomerase | K7V516_MAIZE | 62198 | 7.05 | 64 | 41 | 95 |
| 4-hydrox-7-methoxy-3-oxo-3,4 | P49235_MAIZE | 64196 | 6.22 | 68 | 42 | 92 |
| Dihydro-2H-1-4-benzoxazin Malic enzyme | O50015_MAIZE | 71825 | 7.30 | 68 | 50 | 90 |
| Diacylglycerol kinase | C0PCE8_MAIZE | 77215 | 8.00 | 73 | 52 | 85 |
| Inositol1,3,4,5,6-pentakisphosphate | A6YH14_MAIZE | 48935 | 7.24 | 47 | 38 | 83 |
| Glucose-6-phosphate isomerase cytosolic | P49105_MAIZE | 62198 | 7.05 | 64 | 41 | 95 |
| Putative inosine-uridine hydrolase | Q6PPF8_MAIZE | 35123 | 6.15 | 32 | 22 | 93 |
| Guanine nucleotide-binding protein Subunit beta | P49177_MAIZE | 62198 | 7.05 | 64 | 41 | 95 |
| 1-deoxy-D-xyluose 5-P reductoisomerase 4-hydroxy- 7-methoxy-3-oxo- | Q9FX27_MAIZE | 51252 | 6.45 | 44 | 37 | 79 |
| Putative dTDP-glucose4,6-dehydratase | A1X8E4_MAIZE | 64644 | 5.88 | 72 | 46 | 86 |
| Putative RUB1 conjugating enzyme | Q6PNA0_MAIZE | 20642 | 8.77 | 21 | 14 | 90 |
| Alpha-1,2-Mannosidase | K7UWA5_MAIZE | 71623 | 6.71 | 66 | 45 | 89 |
| Cis-zeatin O-glucosyltransferase | Q8RXA5_MAIZE | 50711 | 5.33 | 43 | 30 | 85 |
| Glutamate dehydrogenase | B9TST3_MAIZE | 55803 | 5.40 | 62 | 47 | 88 |
| Phospholipase D | A0A096SPA_MAIZE | 91071 | 5.36 | 89 | 62 | 81 |
| Sucrose synthase | Q8L5H0_MAIZE | 91868 | 6.13 | 81 | 47 | 92 |
| Arabinogalactan protein | Q9M715_MAIZE | 27939 | 12.1 | 19 | 19 | 81 |
| DTDP-glucose-4-epimerase | Q6QP37_MAIZE | 44001 | 5.80 | 38 | 34 | 87 |
| 2C-type protein phosphatase protein-16 | A0A060D93_MAIZE | 39638 | 6.33 | 39 | 32 | 91 |
| Xyloglucan endotransglucosylase | Q5JZX2_MAIZE | 30766 | 4.49 | 31 | 18 | 98 |
| 6-Phosphogluconate dehydrogenase decarboxylating | O81238_MAIZE | 53022 | 5.84 | 48 | 33 | 83 |
| Putative beta glycosyltransferase | A1X8E0_MAIZE | 22893 | 6.33 | 22 | 16 | 93 |
| Uroporphyrinogen III methyltransferase | P93628_MAIZE | 44939 | 5.52 | 40 | 30 | 91 |
| Methylthioribose-1-phosphate-isomerase | B6TZD1_MAIZE | 38598 | 5.63 | 41 | 27 | 98 |
| UDP-glucose-4-epimerase | Q7XZQ2_MAIZE | 22893 | 6.71 | 31 | 31 | 86 |
| Peroxidase 2 | Q9FEQ8_MAIZE | 35726 | 5.26 | 46 | 21 | 97 |
| Transpoter Proteins | ||||||
| Potassium transporter | E5LFQ7_MAIZE | 86673 | 8.96 | 83 | 51 | 93 |
| Sulphate transporter | A7YF68_MAIZE | 72177 | 9.58 | 72 | 46 | 87 |
| Inorganic Phosphate transporter | Q5CC71_MAIZE | 60540 | 8.16 | 79 | 34 | 95 |
| Sodium/hydrogen exchanger | Q9ATZ9_MAIZE | 101069 | 4.80 | 8 | 4 | 99 |
| High affinity nitrate transporter | Q0VH25_MAIZE | 20553 | 9.50 | 72 | 46 | 87 |
| Mitochondrial phosphate transporter | O80413_MAIZE | 38633 | 9.42 | 38 | 27 | 87 |
| ADP, ATP carrier protein | BT6C13_MAIZE | 36158 | 10.3 | 39 | 29 | 89 |
| Stress Related Proteins | ||||||
| Hypoxically induced transcript 2 | O81218_MAIZE | 11525 | 7.17 | 12 | 9 | 86 |
| Senescence-associated protein DH | Q5UCF4_MAIZE | 30147 | 8.49 | 14 | 12 | 78 |
| Aquqporin TIP2-3 | Q84RL6_MAIZE | 25116 | 6.20 | 11 | 6 | 52 |
| Sugar starvation induced protein | Q41855_MAIZE | 25043 | 12.1 | 22 | 15 | 72 |
| Pyrabactin resistance-like protein | C0PK92_MAIZE | 20547 | 6.17 | 17 | 17 | 84 |
| Aquaporin PIP2-4 | Q9ATM6_MAIZE | 30302 | 6.59 | 21 | 18 | 78 |
| High mobility group B protein | P93047_MAIZE | 15671 | 5.53 | 24 | 10 | 85 |
| Heat shock protein 101 | Q9S822_MAIZE | 101069 | 5.76 | 112 | 77 | 86 |
| Alcohol dehydrogenase class-P | P06525_MAIZE | 41151 | 5.79 | 43 | 29 | 93 |
| Submergence induced protein SI397 | Q6XPW9_MAIZE | 38965 | 6.37 | 35 | 29 | 90 |
| Aquaporin PIP1-1 | Q41870_MAIZE | 30865 | 9.66 | 22 | 18 | 83 |
| Aquaporin PIP2-5 | Q9XF58_MAIZE | 41283 | 7.83 | 24 | 18 | 98 |
| Defense related proteins | Q41802_MAIZE | 15532 | 8.77 | 12 | 8 | 55 |
| Root Specific Proteins | ||||||
| Root cap-specific protein | K7W6W9_MAIZE | 41283 | 8.35 | 35 | 32 | 82 |
| Rootless concerning crown and seminal lateral roots | A5H451_MAIZE | 24796 | 5.62 | 11 | 11 | 47 |
| Roothairless 1 | Q5YLM3_MAIZE | 100027 | 5.35 | 102 | 63 | 88 |
| Protein root hair Defective 3 homolog | K7UC12_MAIZE | 92200 | 5.99 | 111 | 74 | 89 |
| Protein Shoot Gravitropism 5 | F41P43_MAIZE | 50108 | 9.19 | 51 | 36 | 86 |
| CAPS-like protein 5C1 | B6U300_MAIZE | 16647 | 6.01 | 12 | 4 | 100 |
| Plasma membrane instrinsic protein | Q84RL8_MAIZE | 30657 | 9.05 | 31 | 16 | 97 |
| Outer plastidial membrane protein | P42057_MAIZE | 29958 | 8.44 | 23 | 20 | 95 |
| Casparian strip membrane protein | B6T957_MAIZE | 19995 | 9.98 | 17 | 9 | 93 |
| Hormones | ||||||
| Phytoene Synthase 3 | B0KZ40_MAIZE | 47271 | 8.76 | 61 | 33 | 92 |
| Cytokinin dehydrogenase 1 | O22213_MAIZE | 64883 | 9.60 | 53 | 45 | 74 |
| Cytokinin dehydrogenase 5 | Q67YU0_MAIZE | 60358 | 5.98 | 52 | 32 | 8 |
| Cytokinin dehydrogenase 2 | Q9FUJ3_MAIZE | 55548 | 7.37 | 44 | 37 | 84 |
| ABA-and ripening-inducible-like proteins | Q41730_MAIZE | 18493 | 11.6 | 23 | 19 | 73 |
| Proteins Related to Nitrogen Metabolism | ||||||
| Reactive Intermediate Deaminase A | Q94JQ4_MAIZE | 19803 | 8.74 | 23 | 17 | 95 |
| Heme oxygenase-1 | E5L882_MAIZE | 31566 | 8.66 | 34 | 28 | 94 |
| Ferredoxin | Q9SLP6_MAIZE | 39297 | 8.42 | 54 | 33 | 86 |
| High affinity Nitrate Transporter | Q53CL7_MAIZE | 56637 | 7.84 | 64 | 28 | 95 |
| Non-symbiotic hemoglobin | Q9FY42_MAIZE | 18267 | 6.38 | 26 | 19 | 91 |
| Glutamine synthetase root isozyme-1 | P38559_MAIZE | 39225 | 5.50 | 39 | 27 | 94 |
| Cysteine synthase | P80608_MAIZE | 34184 | 5.81 | 43 | 29 | 84 |
| Glutamine S root isozyme 2 | P38560_MAIZE | 40068 | 5.50 | 37 | 26 | 94 |
| Glutamine s root isozyme 4 | P38562_MAIZE | 38956 | 5.05 | 33 | 25 | 92 |
| Aspartate aminotransferase | B4FUH2_MAIZE | 50150 | 8.50 | 68 | 34 | 92 |
| Amino methyl transferase | A0A096UFY3_ MAIZE | 46792 | 7.56 | 63 | 40 | 97 |
| Glutamine s root isozyme 5 | P38563_MAIZE | 39234 | 5.39 | 36 | 29 | 89 |
| High affinity nitrate transporter | Q0VH26_MAIZE | 21075 | 9.30 | 13 | 12 | 94 |
| Glutamine s isozyme 3 | P38561_MAIZE | 39114 | 5.06 | 27 | 25 | 89 |
| Ferredoxin –NADP reductase | Q41736_MAIZE | 36352 | 8.21 | 39 | 30 | 88 |
| Glutamate Dehydrogenases | Q43260_MAIZE | 43994 | 6.07 | 50 | 27 | 90 |
| Glutamate decarboxylase | B9TST3_MAIZE | 55803 | 5.40 | 62 | 47 | 88 |
| Asparagine synthetase | P49094_MAIZE | 66535 | 5.78 | 57 | 43 | 85 |
| Nitrate reductase [NAD(P)H] | P39871_MAIZE | 26237 | 6.14 | 33 | 19 | 90 |
| Mitochondrial phosphate transporter | O80413_MAIZE | 38633 | 9.42 | 38 | 27 | 87 |
| Cysteine proteinase | Q10716_MAIZE | 40321 | 5.88 | 26 | 27 | 76 |
| Hydroxyproline-rich Glycoproteins | Q42366_MAIZE | 34409 | 10.3 | 6 | 4 | 22 |
| D-alanine ligase | O8RVL2_MAIZE | 238111 | 6.11 | 197 | 143 | 92 |
| Putative nitrous oxide reductase | Q6VUZ8_MAIZE | 24889 | 5.53 | 25 | 11 | 91 |
| Basic leucine zipper | O22763_MAIZE | 45330 | 5.12 | 51 | 27 | 93 |
| Calpain-type cysteine protease | Q8RVL2_MAIZE | 238111 | 6.11 | 197 | 143 | 92 |
| Ubiquitin-40S ribosomal protein | P27923_MAIZE | 17670 | 10.2 | 13 | 12 | 68 |
| Putative peptidyl-prolyl cis-trans | K7UTL4_MAIZE | 69487 | 7.37 | 65 | 45 | 86 |
| Dihydrolipoamide acetyl transferase | Q41737_MAIZE | 9325 | 9.69 | 12 | 6 | 89 |
| Uroporphyrinogen III methyltransferase | P93628_MAIZE | 44939 | 5.52 | 40 | 30 | 91 |
| Methylthioribose-1-phosphate isomerase | B6TZD1_MAIZE | 38598 | 5.63 | 41 | 27 | 98 |
| Cell Wall Proteins | ||||||
| 3-oxoacyl-CoA reductase | Q8L9C4_MAIZE | 35738 | 9.75 | 40 | 27 | 85 |
| Laccase | Q2PAJ1_MAIZE | 64538 | 5.62 | 64 | 38 | 92 |
| Xyloglucan endotransglucosylase | Q5JZX2_MAIZE | 30766 | 4.49 | 31 | 18 | 98 |
| Actin-depolymerizing factor | Q41764_MAIZE | 15889 | 5.29 | 16 | 11 | 84 |
| Beta-tubulin | C4RS46_MAIZE | 9888 | 4.62 | 6 | 4 | 95 |
| Extensin | P14918_MAIZE | 28830 | 10.5 | 3 | 3 | 4.86 |
| Collagen alpha1-like protein | Q6PN99_MAIZE | 11883 | 11.9 | 10 | 7 | 90 |
| Tubulin alpha-1chain | P14640_MAIZE | 49699 | 4.70 | 51 | 34 | 90 |
| a) Accession number according to the UNIPROT database search for Maize proteins b) Molecular weight in (Da), c) pI(pH)-Isoelectric point / pH of proteins d) Number of Peptide e) Theoretical peptides (based on Trypsin digestion and protein sequence cleaved) f) %Coverage of various Proteins detected in LC-MS/MS | ||||||
Table 3:Gravy index of plasma membrane proteins of maize.
| Accession No | Protein Name | Starting Sequence | Gravy | pH/pI | Cellular location |
|---|---|---|---|---|---|
| Q6RXY1_MAIZE | SNF1 related protein kinase | MDGSSKGSGH | -0.352 | 8.32 | Cytoplasm |
| KPRO_MAIZE | Putative receptor protein kinase ZmPK1 | MPRPLAALLS | -0.208 | 6.92 | Membrane |
| Q2A700_MAIZE | Transcription factor MYB42 | MGRSPCCEKA | -0.472 | 8.03 | Nucleus |
| TBA1_MAIZE | Tubulin alpha-1 chain | MRECISIHIG | -0.188 | 4.65 | Cytoskeleton |
| A0A060D7Z4_MAIZE | GRAS transcription factor | MDTLFRSVSL | -0.343 | 5.50 | Nucleus |
| A1XGH3_MAIZE | ALMT1-like protein (Malate trans) | MEIDEMESGV | 0.104 | 8.47 | Cellular M component |
| B4FQB2_MAIZE | AP2-EREBP transcription factor | MCGGAILAEL | -0.732 | 4.91 | Nucleus |
| Q6PNA1_MAIZE | Putative ubiquitin-activating enzyme | MAGGPRRRLG | -0.153 | 7.32 | Cellular M component |
| E2FB_ARATH | Transcription factor E2FB | MSEEVPQQFP | -0.672 | 4.38 | Nucleus/cytoplasm |
| B4FA27_MAIZE | Alpha-galactosidase | MEAAGRLPLL | -0.228 | 6.36 | Cell wall |
| K7TLQ9_MAIZE | Putative MYB DNA-binding domain | MEFIDPWDSQ | -0.382 | 9.53 | ER PM |
| O81229_MAIZE | Ribosomal protein L25 (Fragment) | RPTTLKKARD | -0.676 | 10.62 | Ribosomes |
| A6YDJ4_GIBIN | Translation elongation factor 1-alpha (Fragment) | KTHLNVVVIG | -0.400 | 9.46 | |
| Q8RXB5_MAIZE | Origin recognition complex subunit 5 | DKPSDFVAAL | -0.094 | 6.27 | Nucleus |
| C0PCE8_MAIZE | Diacylglycerol kinase | MDLVGSLLLS | -0.227 | 8.17 | PM |
| A6YH14_MAIZE | Inositol 1,3,4,5,6-pentakisphosphate 2-kinase | MEMDGVLQAA | -0.188 | 7.56 | Nucleus |
| OCS1_MAIZE | Ocs element-binding factor 1 | MSSSSLSPTA | -0.760 | 9.04 | Nucleus |
| O81232_MAIZE | E3 ubiquitin-protein ligase | PRVRFCLHFE | -0.599 | 10.01 | Nucleus |
| MCM33_MAIZE | DNA replication licensing factor MCM3 | MEINEEAMAA | -0.344 | 6.30 | Nucleus |
| B6TPK0_MAIZE | CAK1AT OS | MAIVGGGGSW | 0.369 | 4.15 | Cytosol, Nucleus |
| O48888_MAIZE | ATP sulfurylase | MATQAAFLAG | -0.311 | 9.14 | Chloroplastic |
| Q9ZP60_MAIZE | GST7 protein | MSPPVKILGH | -0.146 | 5.15 | Cytoplasm |
| Q84RL8_MAIZE | Plasma membrane intrinsic protein | MEGKEEDVR | 0.402 | 9.08 | PM and Vacuole |
| Q9ZP61_MAIZE | GST6 protein | MAAAAEVVLL | -0.107 | 5.44 | Cellular component |
| Q9SX02_MAIZE | Replication origin activator 4 (Fragment) | MDVNEEAMAA | -0.234 | 8.49 | Nucleus |
| Q53CL7_MAIZE | High affinity nitrate transporter | MAAVGAPGSS | 0.369 | 8.01 | PM and p-type Vacuole |
| GLNA3_MAIZE | Glutamine synthetase root isozyme 3 | MACLTDLVNL | -0.424 | 5.01 | Cytoplasm |
| Q0VH26_MAIZE | High affinity nitrate transporter | MARQQSVHAL | 0.155 | 9.35 | PM |
| Q6B7Q8_MAIZE | Putative serine/threonine-specific protein kinase | SGYLGAECQE | -0.307 | 8.47 | --- |
| O80413_MAIZE | Mitochondrial phosphate transporter | MALSDRSRES | 0.263 | 9.54 | Mitochondria (IM) |
| Q42366_MAIZE | Hydroxyproline-rich Glycoprotein (HRGP) | MGGSGRAALL | -1.321 | 10.62 | --- |
| K7U915_MAIZE | Phosphoserine phosphatase | MAGLISLRAG | 0.018 | 6.29 | chloroplast/cytoplasm |
| TIP23_MAIZE | Aquaporin TIP2-3 | MVKLAFGSFR | 0.864 | 6.67 | PM |
| Q8RMK8_AZOBR | D-alanine:D-alanine ligase (Fragment) | KALLAPVGVR | -0.061 | 5.30 | Cytoplasm |
| CYSP1_MAIZE | Cysteine proteinase 1 | MAHRVLLLLS | -0.318 | 6.32 | ES, Lysosome, vacuole |
| Q9ATZ9_MAIZE | Sodium/hydrogen exchanger (Fragment) | VFSEVLFFIY | 0.900 | 4.79 | Vacuolar Membrane |
| NIA2_MAIZE | Nitrate reductase [NAD(P)H] (Fragment) | PQKLGLPVGR | -0.473 | 6.60 | Cytosol |
| Gravy index of identified plasma membrane proteins by LCMS/MS technique. The marked colored lines shows the most hydrophobic proteins. Cellular location of proteins searched from UNIPROT database | |||||
Figure 4:Functional classification of expressed proteins under combined stresses in plasma membranes of maize roots. The numbers and percentages of proteins from each functional category assigned on the basis of identified proteins. Proteins were categorized using the criteria of Bevan et al. (1998).
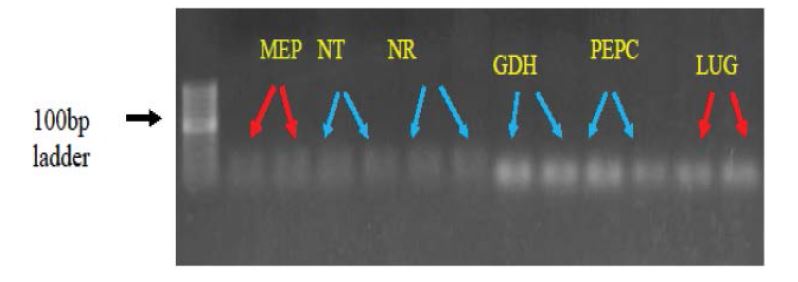
Figure 5:Transcripts of four plasma membrane proteins shows the induction of all proteins in control and stressed plants. Represented with blue arrow-Nitrate transporter (NT), Nitrate Reductase (NR), Gulamine syntetase (GDH) and Phosphenol pyruvate carboxylase (PEPC) RED arrow-MEP and LUG internal control genes of Maize.
shown significant up regulation of gene encoding nitrogen transporter in both the cultivars. Up-regulation of nitrogen transporter gene maintain the N homeostasis in tolerant cultivars, whereas, in salt sensitive cultivars up-regulated gene significantly reducing the N content [21]. Therefore transporter gene may have contributed to the salinity tolerant in both the cultivars. Another study shows higher abundance of transcripts related to high affinity nitrate transporters (NRT2.2, NRT2.3, NRT2.5, and NRT2.6) in tolerant genotype of barley [22]. However, soon after sensing NO-3 concentration in external medium plants respond by activating genes encoding NO-3 transport system and many enzyme systems [23]. In barley plants, Nitrate Reductase is the first enzyme to be involved in assimilation [24-27]. Successively, strong correlation between increased rates of NO-3 uptake and NR activity was observed [28,29]. Among the identified proteins by LCMS/MS was membrane bound NR in stress plants, the presence of NR transcripts in treated plant correlates with them. The transcripts of glutamine synthetase was correspondingly expressed in treated genotypes. Likewise, Li et al., detected the presences of genes of GS1-1 form and its expression in roots and confirms, the assimilation of NH4+ by the glutamine synthetase pathway for the amino acid synthesis. In a similar way, in treated plants transcripts of PEPC (Phosphoenolpyruvate carboxylase) was detected. [30]. A study reported the increase in levels of protein and mRNA for PEPC, by selectively increasing exogenous supply of nitrogen. Also with the steady-state level of PEPC mRNA and the major amino acids, glutamine level increased for 7 hours after nitrogen supply [31]. Thus, q RT-PCR results validates the presence of transcripts in treated and control plants. The results also verify the induction of all the four proteins in treated maize plants and their possible role in tolerance mechanism under multiple stresses. Furthermore, our results correlates with studies of Prinsi et al. They showed the incubation of maize roots for 30h in nitrogen nutrition, enhances the enzymes involved in nitrogen and carbon metabolism [32]. However, Nohzadeh et al. performed real-time PCR analysis, to investigate the correlation between mRNA and protein levels, in the roots of PM of salt-tolerant variety of rice, IR651, for three salt responsive genes (1,4-benzoquinone reductase, a putative remorin protein, and a hyper sensitive induced response protein) [33]. In their results no correlation was detected between the changes in the levels of gene and protein expression. Whereas, our results show correlation between plasma membrane proteins and mRNA level. Though in our study plants were under low-N stress and deficient in nitrogen nutrition but due to the induction of nitrate transporter proteins and other 3-proteins, there is coordinated regulation of interaction of carbon and nitrogen metabolism. Subsequently maintains the homeostasis in various stresses.
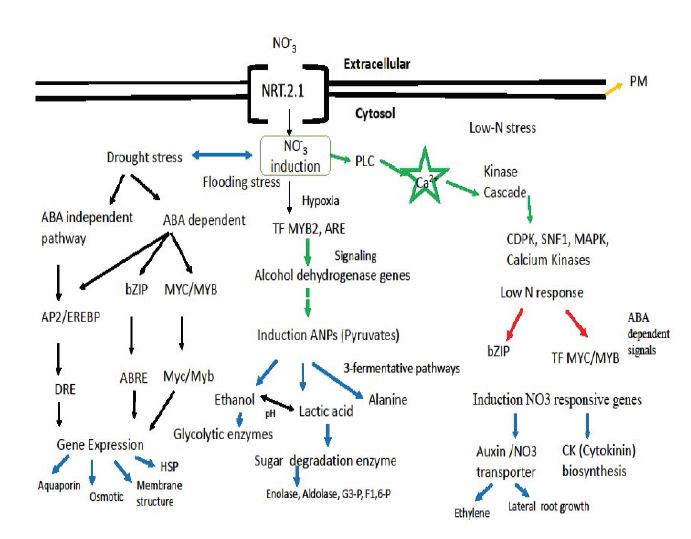
Figure 6:A Model shows the maize plant’s response to multiple abiotic stresses. All the regulations occurring in the root cytosol (to make the model simple, other organelle not shown). Color arrows represent: Green- Signaling Cascade; Red – (+) ve regulation of genes and Blue-N-responsive pathways/metabolism.
Gravy Index of Plasma membrane proteins
The GRAVY is a computational program that evaluate the hydrophilic and hydrophobic properties of proteins along its amino acid sequence. The GRAVY score takes into account the size and the charge of the whole protein and ranges. The GRAVY of the maize roots plasma membrane proteins analyzed ranges from –1.32 to 0.402 [34]. Whereas, positive values referring to hydrophobic proteins. In our roots sample highly hydrophobic proteins is plasma membrane intrinsic protein (Q84RL8_MAIZE) it’s Gravy score +0.4 The table 3, shows the Gravy index of roots plasma membrane proteins along with pI and cellular location of the proteins.
Proposed Model
Therefore, on the basis of our characterization of roots proteins data, qRT-PCR studies, and physiological status of the treated plants in response to various stresses. A model has been proposed that shows the plants response in low-N stress and their combined adversity stress adaptation strategies. The induction of Nitrate transporter proteins that enhanced and involve network of proteins to maintain homeostasis in other two stresses (waterlogging and drought) thus help to acclimatize the maize plants in various abiotic stress conditions has been shown in figure 6.
Conclusion
The maize plants when subjected to various abiotic stresses in combination shows tolerance. The phenotypic observations in treated plants does not exhibited any stress related symptoms. Also, statistical analysis of the mean values of various growth, morphological and physiological parameters in treated and control plants shows no significant difference. Therefore, to understand the reason of tolerance mechanism under multiple abiotic stresses, the roots plasma membrane proteins in treated plants was identify and characterized using LC-MS/MS techniques (in solution). The presence of a large number of integral hydrophilic, hydrophobic and low abundance of proteins were identified in our results. The transcriptional studies validates the role of four proteins (treated plants). Further, the role of other proteins can be validated only after comparing the control roots protein samples with the treated roots. In present context we can assume the role of other characterized proteins of stressed plants, might be due to coordinated regulation and expression of various proteins along with induction of ‘High- Affinity nitrate transporter proteins”. These are involved in sensing and transporting nitrogen in low-N condition, and trigger signaling cascades which in turn activates membrane bound TFs, that initiate transcription of genes for low-N stress, waterlogging and drought stresses. Thus, maintain metabolic homeostasis and counteracting the effects of stress and ameliorate to acclimatize the plants at vegetative stage. Similarly, Scheible et al. observed the direct and indirect consequences of the nitrogen availability on the whole plant metabolism [35].
Solomon S, Qin D, Manning M, Chen Z, Marquis M, et al. (2007) IPCC 2007 Climate change 2007: The physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change Geneva: IPCC Secretariat. [ Ref ]
Bates BC, Kundzewicz ZW, Palutikof J, Wu S (2008) IPCC 2008 Climate change and water. Technical Paper of the Intergovernmental Panel on Climate Change Geneva: IPCC Secretariat 210. [ Ref ]
Mittler Ron (2006) Abiotic stress the field environment and stress combination. Trends in Plant Sci 11: 15-19. [ Ref ]
Atkinson NJ, Urwin PE (2012) The interaction of plant biotic and abiotic stresses: from genes to the field. J of Exp Bot 63: 3523-3543. [ Ref ]
Marmagne AR, Ferro MA, Rolland M, Alcon N, Joyard C, et al. (2004) Identification of new intrinsic proteins in Arabidopsis plasma membrane proteome. Molecular and Cellular Proteomics 3: 675-691. [ Ref ]
Ephritikhine G, Ferro M, Rolland N (2004) Plant membrane proteomics. Plant Physiol biochem 42: 943-962. [ Ref ]
Zhang H Lin, Q Ponnusamy S, kothandaraman N (2007) Differential recovery of membrane proteins after extraction by aqueous methanol and trifluoroethanol. Proteomics 7: 1654-1663. [ Ref ]
Zaidi PH, Mamata Yadav, Singh DK, Singh RP (2008) Relationship between drought and excess moisture tolerance in tropical maize (Zea mays L). Aus J of Crop Sci 1: 8-96. [ Ref ]
Rathore TR, Warsi MZK, Lothrop JE, Singh NN (1996) Production of maize under excess soil moisture waterlogging conditions. 6th Asian Regional Maize Workshop 10-12 Feb 1996 PAU Ludhiana. [ Ref ]
Bradford MMA (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254. [ Ref ]
Ort DR (2001) When there is too much light. Plant Physiol 125: 29-32. [ Ref ]
Chaves MM, Oliveira MM (2004) Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. J Exp Bot 55: 2365-2384. [ Ref ]
Zlatev Z, Lidon FC (2012) An overview on drought induced changes in plant growth water relations and photosynthesis. Emir J Food Agric 24: 57-72. [ Ref ]
Settimi JR, Maranville JW (1998) Carbon dioxide assimilation efficiency of maize leaves under nitrogen stress at different stages of plant development. Commun Soil Sci Plant anal 29: 777-792. [ Ref ]
Bevan M (1998) Analysis of 19Mb of contiguous sequence from chromosome 4 of Arabidopsis thaliana. Nature 391: 485-488. [ Ref ]
Alexandersson E, Saalbach G, Larsson C, Kjellbom P (2004) Arabidopsis plasma membrane proteomics identifies components of transport signal transduction and membrane trafficking. Plant Cell Physiology 45: 1543- 1556. [ Ref ]
Miller AJ, Fan X, Orsel M, Smith SJ, Wells DM (2007) Nitrate transport and signaling. J Exp Bot 58: 2297-2306. [ Ref ]
Krapp A, Fraisier V, Scheible WR, Quesada A, Gojon A, et al. (1998) Expression studies of Nrt2:1Np a putative high affinity nitrate transporter: evidence for its role in nitrate uptake. The Plant Journal 14: 1051-1061. [ Ref ]
Filleur S, Daniel-Vedele F (1999) Expression analysis of a highaffinity nitrate transporter isolated fromArabidopsis thaliana by differential display. Planta 207: 461-469. [ Ref ]
Siddiqi MY, Glass ADM, Ruth TJ, Rufty TW (1990) Studies the uptake of nitrate in barleyI Kinetics of 13NO-3 influx. Plant Physiol 93: 1426-1432. [ Ref ]
Senadheera P, Singh RK, Frans J, Maathuis M (2009) Differentially expressed membrane transporters in rice roots may contribute to cultivar dependent salt tolerance. J Exp Bot 60: 2553-2563. [ Ref ]
Malleswari G, Duo Y, Reddy A, Zhang KC, Holding D, et al. (2014) Identification of differentially expressed genes between sorghum genotypes with contrasting nitrogen stress tolerance by genome-wide transcriptional profiling. BMC Genomics 15: 179. [ Ref ]
Gojon A, Krouk G, Perrine-Walker F, Laugier E (2011) Nitrate transceptor (s) in plants. J Exp Bot 62: 2299-2308. [ Ref ]
Crawford NM (1995) Nitrate: nutrient and signal for plant growth. Plant Cell 7: 859-868. [ Ref ]
Wang R, Tischner R, Gutierrez RA, Hoffman M, Xing X et al. (2004) Genomic analysis of the nitrate response using nitrate reductase-null mutant of Arabidopsis. Plant Physiol 136: 2512-2522. [ Ref ]
Ho CH, Lin SH, Hu HC, Tsay YF (2009) CHL1 functions as a nitrate sensor in plants. Cell 138: 1184-1194. [ Ref ]
Krouk G, Crawford NM, Coruzzi GM, Tsay YF (2010) Nitrate signaling adaptation to fluctuating environments. Curr Opin Plant Biol 13: 266-273. [ Ref ]
Larsson CM, Ingemarsson B (1989) Molecular aspects of nitrate uptake in higher plants. Molecular and Genetic Aspects of Nitrate Assimilation. Oxford Science Publishers, New York NY. [ Ref ]
Jackson WA, Pan WL, Moll RH, Kamprath EJ (1986) Uptake translocation and reduction of nitrate. Biochemical basis of Plant Breeding (C Neyra ed) 2: 73-108. [ Ref ]
Li M, Villemur Hussey R, Silflow PJ, Gantt CD, Snustad JS (1993) Differential expression of six glutamine synthetase genes in Zea mays. Plant Mol Biol 23: 401-407. [ Ref ]
Sugiharto B, Sugiyama T (1992) Effects of Nitrate and Ammonium on Gene Expression of Phosphoenolpyruvate Carboxylase and Nitrogen Metabolism in Maize Leaf Tissue during Recovery from Nitrogen Stress1. Plant Physiol 98: 1403-1408. [ Ref ]
Alfredo PB, Paolo SN, Maurizio P, Espen CL (2009) Evaluation of protein pattern changes in roots and leaves of Zea mays plants in response to nitrate availability by two-dimensional gel electrophoresis analysis. BMC Plant Biol 9: 113. [ Ref ]
Malakshah N, Rezaei SH, Heidari M, Salekdeh MGH (2007) Proteomics reveals new salt responsive proteins associated with rice plasma membrane. Biosci Biotechnol Biochem 71: 2144-2154. [ Ref ]
Kyte J, and Doolittle R F (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157: 105-132. [ Ref ]
Scheible WR (2004) Genome-wide reprogramming of primary and secondary metabolism protein synthesis cellular growth processes and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiology 136: 2483-2499. [ Ref ]