Journal Name: Journal of Pediatrics and Infants
Article Type:Research
Received date:02 March, 2021
Accepted date:27 August, 2021
Published date:03 September, 2021
Citation:Chen Q, Li Z, He Yd (2021) A Risk-Prediction Nomogram for Patients with Second-Trimester Threatened Miscarriage Associated with Adverse Outcomes. J Pediat Infants Vol: 4, Issu: 2 (40-47).
Copyright:© 2021 Chen Q et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Both ultrasound, demographic, biochemical and laboratory markers alone have been analyzed in the previous literatures for the prediction of miscarriage; however, these independent factors have not yet been integrated for analysis. Thus, we performed this analysis to determine the best combination markers to establish a nomogram prediction model for patients presenting with secondtrimester threatened miscarriage and verify its validity prospectively.
Methods: We retrospectively collected information from the patients hospitalized with second-trimester threatened miscarriage and used the logistic regression analyzes to determine the most significant predictive factors associated with miscarriage. While the individualized risk-prediction nomogram model was established based on the predictors’ regression coefficients. The area under the receiver operating characteristic curves and the Hosmer-Lemeshow test were utilized to verify the discrimination and calibration of the prediction model, respectively.
Results: This study demonstrates that gestational weeks, C-reactive protein, vaginal blood loss, premature rupture of membranes, and uterine adenomyosis or adenomyoma were the most significant independent risk factors of the second-trimester threatened miscarriage associated with adverse outcomes.
Conclusion:We can estimate the possibility of second-trimester miscarriage through the nomogram prediction model which has good recognition and calibration.
Keywords: Miscarriage, Nomogram, Second-trimester threatened miscarriage.
Abbreviations: PPROM: premature rupture of membranes; PIH: pregnancy-induced hypertension; PE: preeclampsia; PTD: premature delivery; FGR: fetal growth restriction; NICU: neonatal intensive care unit; BMI: body mass index; PCOS: polycystic ovarian syndrome; LMP: last menstrual period; CRL: crown-rump length; CRP: C-reactive protein; PROM: Premature rupture of membranes; ORs: Odds ratios; ROC: Receiver operating characteristic; PBAC: Pictorial Blood Loss Assessment Chart; AUC: The area under the ROC curves.
Background
Spontaneous miscarriage is one of the most common complications in early pregnancy, affecting 10-20% of all pregnancies, and this rate has increased in recent years [1-5]. Late miscarriage is less common, occurring in 1-2% of pregnancies. The incidence increases further with vaginal bleeding. The vaginal bleeding is usually accompanied by pelvic or paroxysmal lower abdominal pain; these symptoms may disappear after rest and treatment and the pregnancy may continue, the main treatments for threatened miscarriage are bed rest, luteal support, contractile inhibitor and combined estrogen and P4 supplements [6]. However, if the vaginal bleeding increases or the lower abdominal pain intensifies, approximately 14.3- 50% of threatened miscarriage patients will experience a subsequent complete miscarriage [7-10]. More than 80% of miscarriages occur within the first 12 gestational weeks, while relatively few occur after 12 weeks. Vaginal bleeding during pregnancy may be related to adverse fetal and maternal outcomes [11-17].
The factors of late miscarriage are varied, including embryonic or fetal chromosomal abnormalities, environmental immune factors, maternal endocrine dysfunction, maternal comorbidities (for instance, diabetes mellitus, antiphospholipid syndrome), terat-uterus, placental dysplasia and smoking [2,3]. It has been confirmed that experiencing threatened miscarriage may suggest potential placental dysfunction, which may manifest in subsequent pregnancy, leading to poor fetal and maternal outcomes such as second-trimester miscarriage, preterm premature rupture of membranes(PPROM), placenta previa, pregnancy-induced hypertension (PIH)/preeclampsia (PE), premature delivery (PTD), fetal growth restriction (FGR) and neonatal intensive care unit (NICU) admission [18-21].
In recent years, the number of articles studying the predictive factors of spontaneous miscarriage has increased significantly. The overwhelming majority of these available studies assess symptomatic patients. Repeated symptoms can cause maternal physical and psychological distress and anxiety, which may continue all through the whole pregnancy [22]. Moreover, the uncertain prognosis of threatened miscarriage is also challenging to obstetricians. In particular, it is difficult to estimate potential pregnancy outcome based solely on the patients’ initial clinical symptoms [1,2,4,23]. Therefore, the development of early screening and a reliable predictive model to calculate the probability of suffering from spontaneous miscarriage can be of great use in many ways: on the one hand, a predictive outcomes with low risk can reduce depression and worry in patients, but on the other hand, a predictive outcomes with high risk could help identify patients who should receive counselling regarding the likelihood of an ongoing pregnancy or miscarriage as early as possible if they become symptomatic, therefore not prolonging the unnecessary suffering of the patient by rest or treatment. Last but not least, challenges can be mitigated, which may someday lead to potential new therapies, followup scans regularly which was used to evaluate the fetal viability as well as to rule out any uterine malformation and appropriate counselling for such patients [24].
Methods
Data collection
We collected the clinical data of 228 pregnant women diagnosed with second-trimester threatened miscarriage in the development cohort retrospectively who were admitted to our hospital from January 2016 to August 2018, as well as the known pregnancy outcomes, including 33 patients with late miscarriage and 195 patients without late miscarriage. A total of 24 patients with second-trimester threatened miscarriage admitted between September 2018 and November 2018 were included in the validation group, of whom 2 patients suffered from late miscarriage and 22 patients didn’t experience late miscarriage.
Inclusion and exclusion criteria
There are different definitions of ‘threatened miscarriage’ and ‘second trimester’ among the various articles and guidelines. Threatened miscarriage is the diagnosis for the pregnant patient who presents with vaginal bleeding and no cervical dilation or effacement before 20 weeks of gestation. These patients may present with spotting to severe vaginal bleeding of several hours to days in duration [25]. The time span is up to 24 weeks of gestation [26]. In the present study, second-trimester threatened miscarriage occurs during the second trimester (14 to 28 weeks of gestation).
The exclusion criteria were as follows:
(1) Bleeding in the first and third trimester only.
(2) Women who did not participate in follow-up or delivered outside the hospital.
(3) Women who opted for termination.
(4) Known genetic/acquired thrombophilia.
(5) Large leiomyomata distorting the uterine cavity which transvaginal ultrasound shows.
(6) Women admitted to the hospital with fibroid degeneration, torsion of an ovarian cyst pedicle or an acute abdomen.
(7) Women with bleeding originating from an extragenital region or with other causes of vaginal bleeding (e.g., postcoital bleeding, cancer, cervical polyp, vaginitis) and with unknown bleeding histories.
(8) Hydatidiform moles.
Successful treatment criteria included the following:
(1) After proper treatment, vaginal bleeding stopped, there was no abdominal discomfort, and there was no sense of uterine contractions.
(2) Ultrasound reexamination indicated that fetal development was in line with the gestational weeks and fetal heart rate, there was no cervical dilation or effacement.
Ethics statement
This study was approved by the Ethics Committee of Peking University First Hospital (ethics approval number: 2019-139) prior to the commencement of the study, and written informed consent was provided by the participants. The study was conducted according to the principles of the Declaration of Helsinki and its amendments.
Predictors
We enrolled the baseline clinical data of the patients with threatened miscarriage. More than one hundred variables were obtained, including general information (gestational weeks, race/nationality, maternal age, admission temperature, admission pulse, maternal height, pregestational weight, pregestational body mass index (BMI), use of assisted reproductive technology, singleton or multiple pregnancy, prenatal diagnosis, admission treatment, pregnancy complications, uterine malformation, polycystic ovarian syndrome (PCOS), leiomyoma, uterine adenomyosis or adenomyoma, cervical surgical history, cervical lesions (including cervical incompetence), previous obstetric history (including history of miscarriage and induced abortion, previous preterm delivery, previous term delivery, history of obstetric abnormality and previous ectopic pregnancy), type of delivery and pregnancy outcome. Specific information was also collected, to include characteristics of threatened miscarriage, such as several admissions for second-trimester threatened miscarriage , abnormal sonographic features, specific symptoms (e.g., vaginal bleeding or/and abdominal pain), and vaginal blood loss, Other data included laboratory test results (routine blood examination, urine culture, vaginal secretion culture, cervical secretion culture, microflora assessment of the vaginal sample, procalcitonin and placental pathology). All baseline data were recorded in a Microsoft Excel.
The measurement of vaginal blood loss during pregnancy after admission is objective. Vaginal blood loss was measured after admission using the weighing method (multiply the wet weight minus the dry weight by 0.95). Since the subjective estimation of vaginal blood loss before admission is always incorrect and objective assessment may be unrealistic. We quantified the amount of vaginal blood loss at the initial visit [27,28].
Gestational weeks were calculated from the first day of the last menstrual period (LMP) and confirmed according to the first two ultrasound reports. If the embryonic bud length and crown-rump length (CRL) dating differed by more than 7 days or multiples of 7 days from the LMP dating, the gestational week was checked based on the embryonic bud length and CRL dating [29,30]. If the patient received assisted reproductive technology, the gestational week was estimated based on the transplanted date, LMP and ultrasound scan.
C-reactive protein (CRP) is sharply elevated in the plasma when the body is infected or damaged by tissue. It activates and strengthens the phagocytosis of phagocytic cells to regulate and remove the invading organism or damaged, necrotic or apoptotic tissue cells.
Vaginal blood loss is a term referring to the amount of vaginal bleeding since the initial hospital visit.
Premature rupture of membranes (PROM) is a term referring to the natural rupture of membranes before delivery.
Uterine adenomyosis is defined as a benign lesion of the endometrium that invades the muscular layer and can be diagnosed by ultrasound, clinical examination and the patient’s previous medical history. A few adenomyosis lesions show localized growth to form nodules or clumps, similar to intermural myoma, called adenomyoma.
Statistical analysis
We came to a conclusion that the baseline data in the research were not normally distributed by statistics analysis. Thus, these measurement data were presented as medians (25th-75th% quartiles), and the count data were presented as frequencies (percentages). We applied the Mann-Whitney U test to analyze the measurement data and the chi-square test to analyze the count data respectively. In addition, we assessed the associations of the variables in regard to late miscarriage in the development group by univariate and multivariate logistic regression analyses. Variables which were shown to be statistically significant in the univariate analysis were incorporated into the multivariate logistic regression analysis, and then the independent risk factors were selected through a forward stepwise process for model fitting. The most significant independent risk factors, a total of five, were screened and applied to establish a model for predicting the occurrence of miscarriage. Odds ratios (ORs) were calculated to estimate the relative risk of adverse consequences. It is believed that probability value (P-value) <.05 was statistically significant. Since the OR indicates the magnitude of the effect, any choice of OR cut-off ≥2.0 represents clinically significant risks and enhances the relevance of the research.
We used the ROC curve calculation cutoff value corresponding to the largest value on the Youden index to group the continuous variables, such as CRP. We eventually divided CRP into two groups, CRP≤8 mg/L and CRP>8 mg/L. Moreover, we used the optimal scale regression grouping to segment the continuous variables, such as gestational weeks, and divided the data into three groups according to the closed scores. We eventually divided the gestational weeks into three groups: ≤18 weeks, between 19 and 23 weeks and ≥24 weeks.
We developed an individualized predicted model of subsequent miscarriage associated with second-trimester threatened miscarriage on the basis of regression coefficients corresponding to five predictors screened from multivariate analysis. We assessed the predictive model according to its discrimination and calibration. The discrimination of the predictive model is the ability to differentiate between women with second-trimester threatened miscarriage that developed into miscarriage from those didn’t develop into miscarriage. The receiver operating characteristic (ROC) analysis was applied for the assessment of the diagnostic performance. The ROC curve was drawn by drafting the sensitivity against 1-specificity of various cut-off values. The area under the ROC curves (AUC) refers to the probability of the trial statistically, thus differentiating normal condition from abnormal condition. The AUC value ranges from 0.5 to 1.0, when the AUC value is 1 which means the trial is perfect, while the value of 0.5 represents the trial is not ideal. The closer the AUC value is to 1, the better the discriminative ability of the predictive model. Generally speaking, it is believed that the AUC value of the predictive model between 0.5 and 0.75 is acceptable, and the AUC value >0.75 suggests that the model exhibits remarkable differentiation [27]. The AUC value of the development group was 0.882, indicating that the discrimination ability of predictive model is good enough.
The degree of calibration represents the consistency between the predicted probabilities and actual probabilities. The small P-value (P<.05) suggests that the calibration of the predictive model is not good enough. The large P-value on the Hosmer-Lemeshow test indicates that there is no compelling proof that the predictive model lacks fitting degree. The P-value of Hosmer-Lemeshow test in the development group was 0.923; therefore, the calculated probability derived by predictive model was highly in accordance with the observed probability, indicating that the calibration of the predictive model in the development group was perfect and the predictive model had a strong coordination. A validation group of 24 patients was applied for further verification. The diagnostic performance was determined based on accuracy, sensitivity and specificity.
IBM SPSS statistics 23 and R software (Version 3.5.0, USA) were applied to analysis statistics. P ≤.05 implied that the differences were obviously statistically significant.
Results
Patient demographics
In the research, complete data were available for 252 patients whose subsequent outcomes were known and who were enrolled in the final analysis, including 228 patients in the development set and 24 patients in the verification set respectively. The overall second-trimester miscarriage rate was 14.4%, with an 85.6% survival rate at the end of pregnancy.
Table 1 showed descriptive statistics for the continuous variables of the clinical presentation at the first visit. Gestational weeks, vaginal blood loss, white blood cell count and CRP were significantly associated with second-trimester miscarriage. A P-value of <.05 was considered statistically significant.
Contrast of all the indicators showed that there is no obviously statistical difference in terms of the patients’ essential information, characteristics of second-trimester threatened miscarriage, auxiliary examination and other baseline data between two groups in this model.
| Development group (n=228) | Validation group (n=24) | Z/X² | P-value | |
|---|---|---|---|---|
| Gestational weeks | 6.319 | 0.000 | ||
| ≤18 | 42(0.18) | 2(0.08) | ||
| 19-23 | 98(0.43) | 9(0.38) | ||
| ≥24 | 88(0.39) | 13(0.54) | ||
| Age (years) | 33(30-35) | 35(32-36) | 0.933 | 0.351 |
| Admission temperature (°C) | 36.7(36.5-37) | 36.65(36.5-36.95) | 0.545 | 0.586 |
| Admission pulse (bpm) | 88(82-96) | 85.5(80-88.75) | 0.293 | 0.77 |
| Height (cm) | 162(160-165) | 160(158-163.75) | 0.487 | 0.628 |
| Pregestational weight (kg) | 60(53.63-66.88) | 61.5(54.13-68) | 1.601 | 0.111 |
| Vaginal blood loss (mL) | 0(0-3.75) | 0(0-14.5) | 1.883 | 0.068 |
| WBC (×109/L) | 10.12(8.78-11.52) | 9.57(8.20-10.58) | 2.832 | 0.005 |
| NE% | 76.8(72.925-81.2) | 77.15(71.425-82.175) | 1.69 | 0.092 |
| CRP (mg/L) | 3.028 | 0.004 | ||
| ≤8 | 185(0.81) | 17(0.71) | ||
| >8 | 43(0.19) | 7(0.29) | ||
| NE%, neutrophilic granulocyte percentage; WBC, white blood cell count; CRP, C-reactive protein. | ||||
Table 1: Baseline continuous characteristics of the development and validation groups.
Nomogram development
The consequence of univariate logistic regression analysis indicated that the statistically significant indicators were gestational weeks, vaginal bleeding, uterine contraction, the premature rupture of membranes, admissions for secondtrimester threatened miscarriage, vaginal blood loss, WBC count, CRP, bacterial vaginosis and uterine adenomyosis or adenomyoma (P<.05).
We incorporated variables of statistical significance in the logistic univariate regression analysis into the nonconditional multivariate logistic regression analysis. The most significant variables for determining the outcome were gestational weeks, CRP, vaginal blood loss, the presence or absence of the PROM, and uterine adenomyosis or adenomyoma. The final results of the logistic regression analysis are listed in table 2 (P<.05). The logistic regression model can be expressed as:
----------equation---------Prefers to the probability of second-trimester miscarriage; PROM and adenomyosis or adenomyoma have a value of 1 if present and 0 if absent. Otherwise, the predictive model can be represented as follows to obtain the probability of miscarriage. This model is derived from the development group.
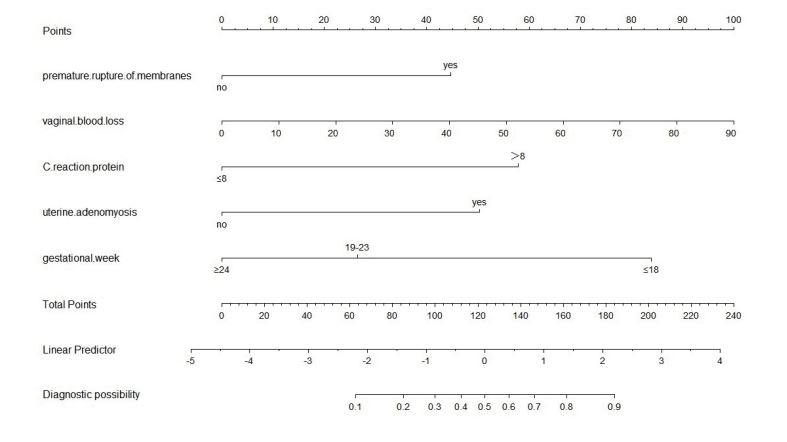
----------equation---------According to the multivariate regression analysis, the five independent risk factors were incorporated into nomogram prediction pattern. The ROC analysis was used for the development and validation groups. The ROC curve was drawn by drafting the sensitivity (true positive rate) of various cut-off values against 1-specificity (false positive rate). The sensitivity represents true positive rate and one minus specificity denotes false positive rate. The AUC can be statistically accounted for as the likelihood of the test, thus differentiating normal conditions from abnormal conditions. We created a personalized nomogram predictive pattern for second-trimester threatened miscarriage according to the regression coefficients (Figure 1). The nomogram predictive model is employed as follows: we are able to find the corresponding points to each predictor in the nomogram; the sum of the scores is referred to the final score, and the predicted risk corresponding to the total score is the likelihood of second-trimester threatened miscarriage associated with second-trimester miscarriage.
Nomogram verification
The verification of the predictive model was in accordance with the evaluation of its discrimination and calibration. The distinction of the nomogram predictive model refers to its ability to distinguish patients with threatened miscarriage from patients with miscarriage. The calibration of the prediction model refers to the consistency between the predicted probability and the actual observed probability.
We plotted the ROC curves for the prediction probability and calculated the AUC values of both groups [28]. The ROC curves were applied to compare the AUC values of the independent risk factors from the multivariate logistic analysis (Table 3).
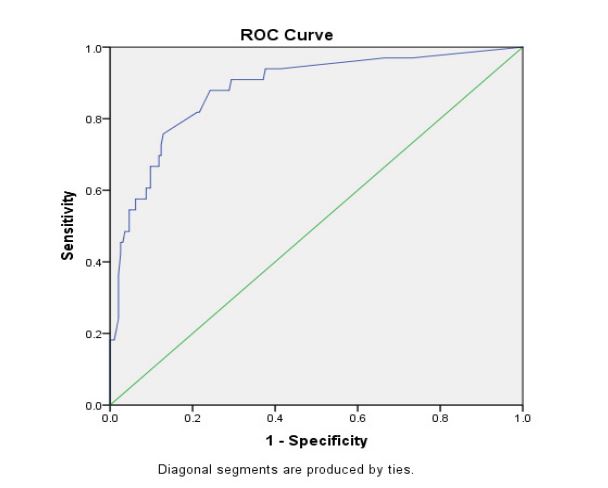
In a retrospective analysis of 228 patients in the development group, the AUC was 0.882 when the probability cutoff point was set at 0.175. The Youden index was 0.637. The sensitivity of this model is 87.9% and the specificity is 75.8% in the development group, and the sensitivity is 100% and the specificity is 91% in the validation group respectively.
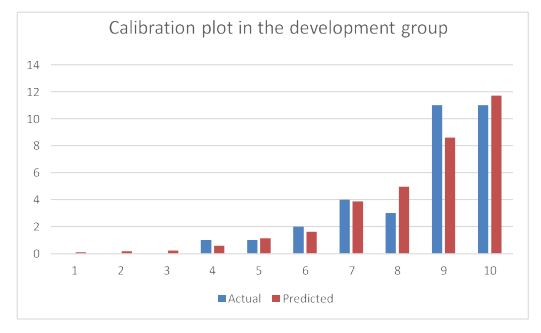
The AUC value for second-trimester miscarriage risk in the development group was 0.882 (Figure 2), indicating that the predictive model has an outstanding distinguishing force). The P-value of the Hosmer-Lemeshow test in the development group was 0.923 (Figure 3). Thus, the predicted probability of the predictive model was basically in accordance with the actual probability, suggesting that the predictive model has a high accuracy performance and that the calibration of the predictive model was ideal enough.
Discussion
The conclusion drawn from this research are basically in accordance with those of previous studies. We have successfully established and verified a prediction model for the patients admitted for second-trimester threatened miscarriage.
As far as we know, this is the first research to develop a model for patients presenting with threatened late miscarriage. Previously published literature has illuminated the associated factors, but has not developed a prediction model. The literature search was conducted rigorously. We took careful note of the quality assessment of the studies and collected information and factors closely associated with miscarriage and consider these factors as clinical data to be collected from patients. Our model is statistically reliable which is based on the fact that we prospectively validated it externally.
We have been aware that internal and external validation is a vitally important step of predictive model establishment, since this process can clarify the effectiveness of models which we developed. We externally validated our development model before we apply it to clinical practice.
Past research has utilized the modified Pictorial Blood Loss Assessment Chart (PBAC) to estimate vaginal blood loss at the initial visit [29]. Our study quantified the amount of vaginal blood loss using a weighing method at presentation, which is more precise.
Our prediction model showed great potential to be utilized in outpatient service for patients who desire personalized probability assessment of miscarriage. The model emphasizes that the five factors in the nomogram prediction model are those clinicians use to prepare for patients with threatened miscarriage in daily clinical practice. These risk factors are objective and not dependent upon patients’ subjective statement.
It is considered that patients who are admitted to the hospital for threatened miscarriage wonder their final pregnancy outcome. No model can predict the pregnancy outcome with absolute certainty at present. Nevertheless, the likelihood of second-trimester miscarriage could be predicted well by applying this model to clinical practice. It could help some patients feel less anxious and fearful. However, it will always bring dispensable fear and anxiety for patients whose predictive model represents false positives. There is no established, successful treatment, in spite of all kinds of approaches have been tried [5,30]. What we can do for patients who presents as threatened miscarriage is symptomatic treatment.
The nomogram chart in this study is not only suitable for outpatients and inpatients with signs of second-trimester threatened miscarriage but also for the treatment of patients with a high risk of miscarriage. The nomogram chart is a useful supplement in clinical work and has positive clinical implications in decision-making for patient diagnosis and treatment.
This study has several limitations that need to be improved in the future. This is a retrospective study that cannot avoid selection bias. The clinical data of the development and validation group was collected from a single center, and evidence is still needed from other centers for validation. Moreover, the sample sizes were not large enough. Further studies are needed that include a larger sample size, are ongoing and involve the implementation of clinical interventions based on the results of the study.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR (95%CI) | P-value | OR (95%CI) | P-value | |
| Gestational weeks | 0.771(0.696-0.853) | <.001 | 0.734(0.646-0.834) | <.001 |
| Vaginal bleeding | 2.637(1.246-5.582) | .011 | NA | |
| Uterinecontractions | 0.418(0.198-0.883) | .022 | NA | |
| Premature rupture of membranes | 5.888(2.125-16.316) | .001 | 4.836(1.412-16.651) | .012 |
| Admissions for second-trimester threatened miscarriage | 3.254(1.493-7.095) | .003 | NA | |
| Vaginal blood loss | 1.005(1.000-1.010) | .043 | 1.004(1.000-1.008) | .032 |
| White blood cell count | 1.191(1.032-1.375) | .017 | NA | |
| C-reactive protein | 1.082(1.036-1.130) | <.001 | 1.119(1.060-1.181) | <.001 |
| Bacterial vaginosis | 9.65(1.548-60.161) | .015 | NA | |
| Gestational diabetes mellitus | 0.063(0.008-0.468) | .007 | NA | |
| Uterine adenomyosis or adenomyoma | 5.194(1.673-16.125) | .004 | 6.904(1.748-27.268) | .006 |
| OR, odds ratio; CI, confidence interval; NA, not available. | ||||
Table 2: Univariate and multivariate logistic regression models in the development group.
| Development group | |||
|---|---|---|---|
| AUC | 95%CI | P-value | |
| Nomogram variable | 0.882 | 0.824-0.940 | <.001 |
| Gestational weeks | 0.756 | 0.663-0.849 | <.001 |
| Premature rupture of membranes | 0.595 | 0.481-0.710 | .08 |
| Vaginal blood loss | 0.692 | 0.581-0.802 | <.001 |
| C-reactive protein | 0.690 | 0.585-0.796 | <.001 |
| Uterine adenomyosis or adenomyoma | 0.57 | 0.457-0.684 | .196 |
| ROC, receiver operating characteristic; AUC, area under the curve; CI, confidence interval. | |||
Table 3: The AUCs of the ROC curves for the nomogram and variables from the logistic regression model in the development group.
Figure 1:Nomogram to predict the probability of second-trimester miscarriage in patients with second-trimester threatened miscarriage.
Figure 2:ROC curve for validating the discrimination power of the nomogram.
Figure 3:Calibration plot of the nomogram in the development group.
Conclusion
In conclusion, we have developed an individualized nomogram prediction model that indicates the likelihood of second-trimester miscarriage. We can calculate the probability of second-trimester miscarriage rapidly by the formula, it is conductive to early identify and screen high-risk patients and take treatment measures as early as possible. All in all, establishing a prediction model which could reliably predict the risk of second-trimester miscarriage is of capital importance. Further retrospective studies with rigorous quality control and methodologies are needed.
Declarations
Ethical approval and consent to participate
This study was approved by the Ethics Committee of Peking University First Hospital [protocol No.139/2019] prior to the commencement of the study, and written informed consent was provided by the participants. The study was conducted according to the principles of the Declaration of Helsinki and its amendments.
Consent for publication
Written informed consent has been obtained from the patient for publication of this Research article. A copy of the written consent is available for check by the Editor of BMC pregnancy and childbirth, if needed.
Availability of data and material
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
Funding
None.
Authors’ contributions
ZL: Data Collection, Manuscript writing; Yd H: Project development; QC: provided critical revisions of the report for important intellectual content. All authors participated in the care of the patient and approved the final version of the manuscript for submission.
Acknowledgements
The authors claim that they have no conflict of interests. This manuscript has not been published anywhere. This study did not receive any grant from funding agencies.
Pillai RN, Konje JC, Tincello DG, Potdar N (2016) Role of serum biomarkers in the prediction of outcome in women with threatened miscarriage: a systematic review and diagnostic accuracy meta-analysis. Hum Reprod Update 22: 228-239. [ Ref ]
Jayasena CN, Abbara A, Izzi-Engbeaya C, Comninos AN, Harvey RA, et al. (2014) Reduced levels of plasma Kisspeptin during the antenatal booking visit are associated with increased risk of miscarriage. J Clin Endocrinol Metab 99: E2652-E2660. [ Ref ]
Morley LC, Simpson N, Tang T (2013) Human chorionic gonadotrophin (hCG) for preventing miscarriage. Cochrane Database Syst Rev 31: CD008611. [ Ref ]
Hanita O, Roslina O, Azlin MI (2012) Maternal level of pregnancyassociated plasma protein A as a predictor of pregnancy failure in threatened abortion. Malays J Pathol 34: 145-151. [ Ref ]
Hannan NJ, Bambang K, Kaitu’u-Lino TJ, Konje JC, Tong S (2014) A bioplex analysis of cytokines and chemokines in first trimester maternal plasma to screen for predictors of miscarriage. PLoS One 9: e93320-e933207.[ Ref ]
Günzel-Apel A, Urhausen C, Wolf K (2012) Serum progesterone in pregnant bitches supplemented with progestin because of expected or suspected luteal insufficiency. Reprod Domest Anim 47: 55-60.[ Ref ]
Gary F, Cunningham (2003) Williams Obstetrics, 21st Edition. Journal of Midwifery & Womens Health 48: 369-369.[ Ref ]
Everett C (1997) Incidence and outcome of bleeding before the 20th week of pregnancy: Prospective study from general practice. BMJ 315: 32-34.[ Ref ]
Weiss JL, Malone FD, Vidaver J (2004) Threatened abortion: A risk factor for poor pregnancy outcome, a population-based screening study. Am J Obstet Gynecol 190: 745-750.[ Ref ]
Basama FM, Crosfill F (2004) The outcome of pregnancies in 182 women with threatened miscarriage. Arch Gynecol Obstet 270: 86-90.[ Ref ]
Batzofin JH, Fielding WL, Friedman EA (1984) Effect of vaginal bleeding in early pregnancy on outcome. Obstet Gynecol 63: 515-518.[ Ref ]
Funderburk SJ, Guthrie D, Meldrum D (1980) Outcome of pregnancies complicated by early vaginal bleeding. Br J Obstet Gynaecol 87: 100-105. [ Ref ]
Hertz JB, Heisterberg L (1985) The outcome of pregnancy after threatened abortion. Acta Obstet Gynecol Scand 64: 151-156.[ Ref ]
Mulik V, Bethel J, Bhal K (2004) A retrospective population-based study of primigravid women on the potential effect of threatened miscarriage on obstetric outcome. J Obstet Gynaecol 24: 249-253. [ Ref ]
Sipila P, Hartikainen Sorri AL, Oja H, Von WL (1992) Perinatal outcome of pregnancies complicated by vaginal bleeding. Br J Obstet Gynaecol 99: 959-963. [ Ref ]
Strobino B, Pantel SJ (1989) Gestational vaginal bleeding and pregnancy outcome. Am J Epidemiol 129: 806-815. [ Ref ]
Wijesiriwardana A, Bhattacharya S, Shetty A, Smith N, Bhattacharya S (2006) Obstetric outcome in women with threatened miscarriage in the first trimester. Obstet Gynecol 107: 557-562.[ Ref ]
Dadkhah F, Kashanian M, Eliasi G (2010) A comparison between the pregnancy outcome in women both with or without threatened abortion. Early Hum Dev 86: 193-196.[ Ref ]
.Petriglia G, Palaia I, Musella A, Marchetti C, Antonilli M, et al. (2015) Threatened abortion and late-pregnancy complications: a case-control study and review of literature. Minerva Ginecol 67: 491-497.[ Ref ]
Ozdemirci S, Karahanoglu E, Esinler D (2015) Influence of threatened miscarriage on pregnancy and early postpartum period: A case-control report. J Matern Fetal Neonatal Med 28: 1186-1189.[ Ref ]
Evrenos AN, Cakir Gungor AN, Gulerman C, Cosar E (2014) Obstetric outcomes of patients with abortus imminens in the first trimester. Arch Gynecol Obstet 289: 499-504.[ Ref ]
Qianhua Xu, Juan Chen, Zhaolian Wei (2017) Sex Hormone Metabolism and Threatened Abortion. Med Sci Moint 23: 5041-5048.[ Ref ]
.Al Mohamady M, Fattah GA, Elkattan E, Bayoumy R, Hamed DA (2016) Correlation of Serum CA-125 and Progesterone Levels with Ultrasound Markers in The Prediction of Pregnancy Outcome in Threatened Miscarriage. Int J Fertil Steril 9: 506-511. [ Ref ]
Tong S, Ngian GL, Onwude JL, Permezel M, Saglam B, et al. (2012) Diagnostic accuracy of maternal serum macrophage inhibitory Cytokine-1 and pregnancy-associated plasma protein-a at 6–10 weeks of gestation to predict miscarriage. Obstet Gynecol 119: 1000-1008.[ Ref ]
Coppola PT, Coppola M (2003) Vaginal bleeding in the first 20 weeks of pregnancy. Emerg Med Clin North Am 21: 667-677.[ Ref ]
Webster K, Eadon H, Fishburn S, Kumar G; Guideline Committee (2019) Ectopic pregnancy and miscarriage: diagnosis and initial management: summary of updated NICE guidance. BMJ 367: l6283. [ Ref ]
Niu XK, He WF, Zhang Y, Das SK, Li J, et al. (2017) Developing a new PIRADS v2-based nomogram for forecasting high-grade prostate cancer. Clin Radiol 72: 458-464.[ Ref ]
Harrell FE Jr, Califf RM, Pryor DB, Lee KL, Rosati RA (1982) Evaluating the yield of medical tests. JAMA 247: 2543-2546.[ Ref ]
Mukri F, Bourne T, Bottomely C (2008) Evidence of early first trimester growth restriction in pregnancies that subsequently end in miscarriage. BJOG 115: 1273-1278.[ Ref ]
Balogun OO, da Silva Lopes K, Ota E, Takemoto Y, Rumbold A, et al. (2016) Vitamin supplementation for preventing miscarriage. Cochrane Database Syst Rev 2016: CD004073. [ Ref ]