Journal Name: Journal of Pediatrics and Infants
Article Type:Research
Received date:19 February, 2021
Accepted date:05 July, 2021
Published date:12 July, 2021
Citation:Kil HR, Kim HJ, Choi EH (2021) Clinical Implications of Iron Deficiency Anemia and Hepcidin Values in Kawasaki Disease. J Pediat Infants Vol: 4, Issu: 2 (14-20).
Copyright:© 2021 Kil HR et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Although the relationship between hepcidin expression and outcome of Kawasaki disease (KD) has been reported, the clinical implications of hepcidin and iron deficiency anemia (IDA) in KD are not fully known. The aim of this study is to evaluate the serial changes of hepcidin and IDA markers according to the clinical stages of KD and to assess whether these parameters can predict the clinical outcome of KD.
Methods: We prospectively recruited 50 children with KD and 47 children in febrile and afebrile control groups. All blood samples including hepcidin, interleukin (IL)-6, tumor necrosis factor (TNF)-α and IDA parameters were analyzed before and after intravenous immunoglobulin (IVIG) infusion, and in the convalescent phase in KD. Informed consent was followed in this study.
Results:Of 50 patients with KD, 19 patients (34.0%) were diagnosed with IDA and 8 patients (21.1%) were diagnosed as IDA in the febrile controls (p=0.088). At presentation, serum hepcidin levels were significantly higher in children with KD than those in febrile controls (p=0.001) and hepcidin levels peaked after IVIG treatment in the subacute phase (p=0.001). Iron and transferrin saturations in patients with acute phase KD were significantly lower than those in controls (p=0.001). Also, serum hepcidin levels in patients with KD were negatively associated with the levels of total iron binding capacity (r=- 0.583, p=0.024). No statistical differenceswere found in IDA parameters, hepcidin, IL-6 and TNF-α levels between KD patients with and without coronary artery lesion (CAL).
Conclusion:Our study suggests that IDA parameters and hepcidin levels do not contribute to the development of coronary artery complications or IVIG resistance, although IDA was more common in patients with acute phase KD and high hepcidin levels were found in patients with acute and subacute phase KD.
Keywords: Anemia, Iron deficiency, Hepcidin, Kawasaki disease.
Abbreviations: KD: Kawasaki disease; IDA: Iron deficiency anemia; CAL: Coronary artery lesion; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; HDL-C: high density lipoprotein-cholesterol; IVIG: intravenous immunoglobulin; NT-proBNP: N-terminal pro-brain natriuretic peptide; TIBC: total iron binding capacity.
Abstract
Background: Although the relationship between hepcidin expression and outcome of Kawasaki disease (KD) has been reported, the clinical implications of hepcidin and iron deficiency anemia (IDA) in KD are not fully known. The aim of this study is to evaluate the serial changes of hepcidin and IDA markers according to the clinical stages of KD and to assess whether these parameters can predict the clinical outcome of KD.
Methods: We prospectively recruited 50 children with KD and 47 children in febrile and afebrile control groups. All blood samples including hepcidin, interleukin (IL)-6, tumor necrosis factor (TNF)-α and IDA parameters were analyzed before and after intravenous immunoglobulin (IVIG) infusion, and in the convalescent phase in KD. Informed consent was followed in this study.
Results:Of 50 patients with KD, 19 patients (34.0%) were diagnosed with IDA and 8 patients (21.1%) were diagnosed as IDA in the febrile controls (p=0.088). At presentation, serum hepcidin levels were significantly higher in children with KD than those in febrile controls (p=0.001) and hepcidin levels peaked after IVIG treatment in the subacute phase (p=0.001). Iron and transferrin saturations in patients with acute phase KD were significantly lower than those in controls (p=0.001). Also, serum hepcidin levels in patients with KD were negatively associated with the levels of total iron binding capacity (r=- 0.583, p=0.024). No statistical differenceswere found in IDA parameters, hepcidin, IL-6 and TNF-α levels between KD patients with and without coronary artery lesion (CAL).
Conclusion:Our study suggests that IDA parameters and hepcidin levels do not contribute to the development of coronary artery complications or IVIG resistance, although IDA was more common in patients with acute phase KD and high hepcidin levels were found in patients with acute and subacute phase KD.
Keywords: Anemia, Iron deficiency, Hepcidin, Kawasaki disease.
Abbreviations: KD: Kawasaki disease; IDA: Iron deficiency anemia; CAL: Coronary artery lesion; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; HDL-C: high density lipoprotein-cholesterol; IVIG: intravenous immunoglobulin; NT-proBNP: N-terminal pro-brain natriuretic peptide; TIBC: total iron binding capacity.
Introduction
Kawasaki disease (KD) is an acute systemic vasculitis of medium sized blood vessels that affects multiple systems with a high propensity to coronary arteries [1,2]. In the acute phase of KD, oxidative stress with ongoing inflammation causes the elevation of interleukin (IL)-6, IL-10, and tumor necrosis factor (TNF)-α [3]. There is no single diagnostic test for KD, but patients usually have characteristic laboratory findings such as leukocytosis with neutrophilia, hypoalbuminemia, hyponatremia, abnormal plasma lipids and anemia. Of these, normocytic, normochromic anemia is a common laboratory finding in patients with KD, and decreased hemoglobin count after initial IVIG infusion was known as the risk factor for IVIG-resistant KD [4-6]. However, the incidence and the role of iron deficiency anemia (IDA) in patients with KD is not clearly elucidated.
IDA is the most prevalent micronutrient deficiency in children; hence IDA in KD could be overlooked or regarded as only concomitant finding. In the literature, IDA was associated with the chronic mild inflammatory condition particularly in obese children and adolescents suggesting hepcidin as a potential biomarker [7]. Hepcidin is an acute phase peptide hormone that influences on the immune system. It was mainly produced by the liver secondary to inflammatory stimulus and the excess of iron [8]. A high concentration of hepcidin has been found in anemia of inflammatory disorders, autoimmune diseases such as rheumatoid arthritis, critical illnesses occurring after major operation, severe trauma or sepsis [9].
Recently, accumulating evidence has supported the involvement of hepcidin in anemia of inflammation. Previous reports identified that anemia in KD patients was related to abnormally increased hepcidin levels with functional IDA [10]. However, the relationship between hepcidin levels and the prognosis of KD is less well known. Therefore, we designed this study to elucidate the iron status in patients with KD by evaluating the changing levels of hepcidin and IDA markers in KD patients and control groups and investigated the association between these parameters and clinical outcomes such as coronary artery complications or IVIG resistance in patients with KD.
Materials and Methods
Participants
In this prospective study, we enrolled KD patients who were admitted to the Department of Pediatrics, Eulji University Hospital, Daejeon, Korea between May 2018 and October 2019. The KD group included 24 boys and 26 girls with a mean age of 32.62 ± 18.64 months (2 months to 8 years). Complete KD was diagnosed in the presence of fever lasting for at least 5 days together with at least 4 of the principal clinical features based on American Heart Association criteria [11]. Patients with refractory KD were defined as those who had a persistent fever (≥38.0ºC) that lasted for more than 24 to 36 hours after completion of the initial IVIG infusion. We collected venous blood from patients with KD in the acute phase (before IVIG infusion), subacute phase (2-4 days after IVIG treatment), and the convalescent phase, when ESR was normal (on days 31-36). The agematched controls consisted of 38 children with febrile group and 9 children with afebrile group.
Definitions of CAL and IDA
CALs were defined based on the Z scores of the left main coronary artery, proximal left anterior descending coronary artery, and proximal right coronary artery, and were defined as the Z scores of 2.0 or more. On the basis of the new guidelines of 2017, the Z scores were calculated from each coronary artery dimensions.
Since the lower limit of normal hemoglobin value differs according to age, anemia was defined using the following cut-off value:11.0 g/dL for children under 59 months of age, and 11.5 g/dL for children 5−11 years [12]. Iron deficiency was defined as presenting with low serum iron less than 50 μg/dL and transferrin saturation less than 16%. IDA was diagnosed when the above-stated criteria of both anemia and iron deficiency were met.
Laboratory assessment
Venous blood was sampled and put in a serum separate tube. The samples were centrifuged at 1,000 ×g to for 10 minutes and were stored in a deep freezer until analysis. Hepcidin levels were measured by enzyme-linked immunosorbent assay (ELISA) kit (MyBiosource, San Diego,CA, USA) and the levels of interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) were assayed with an ELISA kit (abcamR, R&D Systems, Cambridge, United Kingdom).
Levels of IDA markers, hepcidin, IL-6 and TNF-α were measured in 97 patients including patients with KD (n=50) and the control groups (n=47). White blood cells counts (WBC), hemoglobin, mean corpuscular volume (MCV), platelet count, sodium, albumin, iron, total iron binding capacity (TIBC), transferrin saturation, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were obtained for all study subjects.
Statistical analysis
We carried out all statistical analyses with SPSS ver. 22.0 (SPSS Korea Data Solutions, Seoul, Korea). Normally distributed continuous data was expressed as mean ± standard deviation. Comparisons of the frequencies between groups were analyzed using chi-square (χ²) exact test. The difference among the groups was assessed using the 2-sample t-test and analysis of variance (ANOVA). Unnormally distributed continuous data were expressed as median. Mann-Whitney-U or Kruskal-Wallis tests were used to assess the differences among the groups. The correlations of serum hepcidin with hemoglobin, ferritin, iron, iron binding capacity and CRP levels were tested with Spearman correlation analysis. A p value less than 0.05 was considered statistically significant.
Results
Characteristics of subjects
The febrile control group consisted of 16 boys and 22 girls with a mean age of 35.97 ± 26.44 months and the afebrile control group included 4 boys and 5 girls with a mean age of 53.0 ± 24.65 months. In the febrile groups, Epstein-Barr virus infection was confirmed in 3 patients and adenovirus was identified in 1 patient by using multiplex reverse transcriptase polymerase chain reaction (RT-PCR). Additionally, bocavirus and rhinovirus were identified in 2 patients and respiratory syncytial virus B and influenza virus B were confirmed in 2 patients in the febrile control group.
Table 1 shows the comparison of IDA profiles between acute-phase KD patients and febrile controls. Of the 50 patients with KD, 19 patients (34.0%) were diagnosed with IDA in the acute phase. Of the 38 patients with febrile controls, 8 patients (21.1%) were diagnosed as IDA (p=0.088). In the afebrile group, only 1 patient (11.1%) fulfilled the definition of IDA.
Of a total 50 patients with KD, 35 (70.0%) patients completely responded to the initial IVIG treatment. Fifteen (30.0%) patients had persistent fever (≥38.0ºC) lasting for more than 24 to 36 hours after the completion of IVIG infusion, and 8 patients (16%) responded to the second IVIG retreatment without additional treatment of corticosteroid. Seven patients (14%) were crossed over methylprednisolone pulse therapy with an additional IVIG infusion. In patients with refractory KD, there were no suspected cases of secondary hemophagocytic lymphohistiocytosis.
Comparison of laboratory findings between KD and control groups
The pre-IVIG iron levels and transferrin saturations were significantly lower in KD patients compared to the control groups (p<0.001 and p=0.001, respectively). We observed high serum ferritin levels in acute phase KD group compared with those in the febrile control group (p=0.001). The serum hepcidin levels were significantly higher in patients with KD compared with febrile controls (p=0.001). There was a significant elevation in serum WBC, ESR, CRP, d-dimer, and NT-proBNP levels in patients with KD compared to controls (Table 2).
Changes of ID markers according to the clinical state in KD
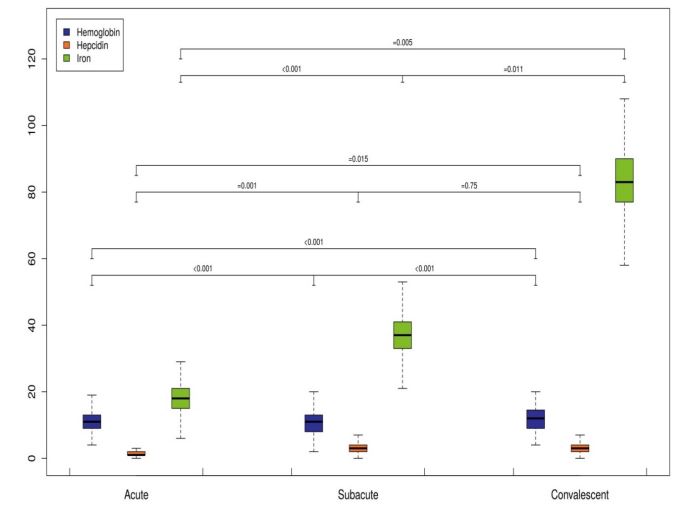
Serum iron levels were lowest in the acute phase of KD and increased significantly in the subacute phase (18.13 ± 11.14 μg/dL before IVIG treatment vs. 36.88 ± 32.34 μg/dL after IVIG treatment, p<0.001). Subsequently, serum iron levels significantly increased to the mean levels of 83.38 ± 39.02 μg/dL in the convalescent phase (Figure 1). However, 2 patients of refractory KD showed further decreased iron levels in the convalescent phase. The serum transferrin saturationand MCV levels were lowest in the acute phase and increased significantly in the convalescent phase (Table 3).
Of the 50 patients with KD, 43 patients (86%) showed significantly decreased hemoglobin levels after IVIG infusion (11.3 ± 0.94 g/dL vs. 10.81 ± 0.84 g/dL, p<0.001). However, 7 patients (14%) showed increased hemoglobin levels above the pre-IVIG level in the subacute phase of KD, all of whom were IVIG responsive. In the convalescent phase, 43 KD patients showed significantly elevated hemoglobin levels compared to those in acute phase (p<0.001). However, 7 patients (14%) revealed a significant decrease in hemoglobin levels in the convalescent phase lower than the pre-IVIG levels, 2 patients of whom were IVIG resistant.
Serum total iron binding capacity (TIBC) level before IVIG treatment was significantly lower compared to the levels of convalescent phase (p<0.001). Serum hepcidin levels were significantly elevated after IVIG treatment in KD patients (1.53 ± 1.36 ng/ml in the acute phase vs 3.09 ± 4.22 ng/mL in the subacute phase, p=0.001). Thereafter, post-IVIG hepcidin levels were subsequently decreased in the convalescent phase.
Comparison of laboratory data of KD patients according to IVIG responsiveness
The initial serum HDL cholesterol levels weresignificantly lower in the IVIG resistance group compared with the IVIG responsive group (26.8 ± 6.3 mg/dL vs. 31.4 ± 6.3 mg/dL, p=0.034). However, there were no differences in hepcidin, hemoglobin, NT-proBNP, CRP, IL-6, and TNF-α levels between these two groups of KD. Serum hepcidin did not have good diagnostic accuracy for predicting IVIG resistance. The area under the receiver operating characteristics (ROC) curve of serum hepcidin for predicting IVIG resistance was 0.44.
| Parameters | Kawasaki disease (n= 50) | Febrile controls (n= 38) | P-value |
|---|---|---|---|
| Age, mon | 32.62 ± 18.64 | 35.97 ± 26.44 | 0.508 |
| Gender (male/female) | 24/26 | 16/22 | 0.582 |
| Weight, kg | 13.90 ± 4.79 | 14.42 ± 6.66 | 0.668 |
| BMI, kg/m² | 16.91 ± 1.92 | 16.14 ± 2.20 | 0.085 |
| Iron deficiency | 25 (50.0%) | 23 (60.5%) | 0.326 |
| Iron deficiency anemia | 19 (34.0%) | 8 (21.1%) | 0.088 |
| Hemoglobin, g/dL | 11.37 ± 0.94 | 11.6 ± 0.91 | 0.153 |
| MCV, fL | 78.38 ± 3.47 | 77.67 ± 5.04 | 0.436 |
| Iron, μg/dL | 19.40 ± 16.89 | 27.51 ± 23.84 | 0.086 |
| TIBC, μg/dL | 247.0 ± 36.33 | 282.49 ± 50.36 | 0.001 |
| Ferritin, μg/L | 179.63 ± 93.34 | 101.36 ± 58.06 | 0.001 |
| Transferrin saturation, % | 7.78 ± 5.97 | 10.03 ± 8.38 | 0.175 |
Table 1: Comparison of demographic and laboratory data between patients with KD and febrile controls.
| Parameters | KD (n = 50) | Febrile controls (n = 38) | Afebrile controls (n = 9) | P-value |
|---|---|---|---|---|
| Hemoglobin, g/dL | 11.37 ± 0.94 | 11.66 ± 0.91 | 12.46 ±1.34 | 0.009 |
| White blood cell, 10³/μL | 12.17 ± 4.18 | 10.80 ±4.13 | 7.76±3.29 | 0.011 |
| Neutrophil, % | 64.26 ± 13.73 | 53.20 ± 16.19 | 45.49 ±9.07 | 0.011 |
| ESR, mm/hr | 65.13 ± 31.36 | 38.32 ± 32.70 | 23.00 ±16.23 | < 0.001 |
| CRP, mg/dL | 7.12 ± 4.49 | 3.64 ± 4.95 | 0.73 ±0.91 | < 0.001 |
| HDL-C, mg/dL | 30.0 ± 7.03 | 36.06 ± 10.47 | 43.42 ±12.41 | < 0.001 |
| Sodium, mEq/L | 135.48 ± 2.20 | 136.87 ± 1.98 | 138.33 ±1.41 | < 0.001 |
| NT-proBNP, pg/mL | 733.72 ± 785.41 | 165.37 ± 203.53 | 94.24 ±81.53 | < 0.001 |
| Albumin, g/dL | 4.09 ± 0.32 | 4.33 ± 0.26 | 4.47 ±0.32 | < 0.001 |
| D-dimer, μg/mL | 1.56 ± 1.12 | 0.88 ± 0.93 | 0.79 ±0.64 | 0.006 |
| Iron, μg/dL | 19.40 ± 16.89 | 27.51 ± 23.84 | 60.56±34.83 | < 0.001 |
| TIBC, μg/dL | 247.0 ± 36.33 | 282.49 ± 50.36 | 320.44 ±44.57 | < 0.001 |
| Ferritin, μg/L | 179.63 ± 93.35 | 101.36 ± 58.06 | 60.76 ±39.80 | < 0.001 |
| Transferrin saturation, % | 7.78 ± 5.97 | 10.03 ± 8.38 | 18.39 ±9.33 | 0.001 |
| Hepcidin, ng/mL | 1.97 (0.76-9.81) | 1.69 (0.01-11.87) | 1.52(1.06-6.41) | 0.001 |
Table 2: Comparison of laboratory values between acute phase KD patients and controls.
| Parameters | Acute phase (n=50) | Subacute phase (n=50) | Convalescent phase (n=50) | P-value |
|---|---|---|---|---|
| Hemoglobin, g/dL | 11.34 ± 0.95 | 10.84 ± 0.86 | 12.05 ± 0.71 | < 0.001 |
| MCV, fL | 78.33 ± 3.48 | 77.95 ± 3.56 | 80.15 ± 3.81 | < 0.001 |
| Iron, μg/dL | 18.13 ± 11.14 | 36.88 ± 32.34 | 83.38 ± 39.02 | 0.011 |
| TIBC, μg/dL | 253.0 ± 41.01 | 255.25 ± 49.49 | 331.00 ± 23.31 | 0.034 |
| Ferritin, μg/L | 194.16 ± 53.68 | 225.86 ± 57.41 | 73.40 ± 50.39 | 0.001 |
| Transferrin saturation, % | 6.77 ± 3.40 | 26.69 ± 5.46 | 20.97 ± 9.83 | 0.019 |
| Hepcidin, ng/mL | 1.53 ± 1.36 | 3.09 ± 4.22 | 2.94 ± 4.65 | 0.001 |
Table 3: Laboratory data of IDA and hepcidin according to the clinical stage of KD.
Figure 1: Boxplots of time dependent changes of ID parameters in patients with KD.
Relationship between clinical parameters in KD patients and development of CAL
The male to female ratio was 4.5 in KD groups with CALs. The percentage of boys were 81.8% in CAL-positive group and 62.5% in CAL-negative group, respectively (p=0.011). In addition, BMI was higher in the CAL-positive group (17.94 ± 1.71 kg/m² vs. 16.61 ± 1.89 kg/m² , p=0.043). There were no statistical differences in regards to the duration of fever, parameters of IDA, hepcidin, IL-6, and TNF-α levels between KD patients with and without CALs (Table 4).
Echocardiographic findings
At presentation, 11 cases (22.0%) among 50 KD patients showed coronary artery dilatation in the acute phase (Table 5). In the convalescent phase, follow-up echocardiography revealed the presence of coronary ectasia in 2 patients (4.0%) with KD. None of patients with KD developed coronary artery aneurysms before or after IVIG treatment. The ratio of the early transmitral flow velocity (E) to the early diastolic mitral annular velocity(e’) ratio measured by tissue Doppler imaging (TDI) was significantly higher in the acute phase of KD compared to the convalescent phase (p=0.001).
Correlations between hepcidin and clinical and laboratory parameters
Serum hepcidin levels in patients with KD were negatively associated with serum TIBC levels (r=-0.583, p=0.024). Age in KD patients was positively correlated with serum leptin levels (r=0.583, p=0.001). In addition, weight and BMI were positively associated with leptin levels in KD patients (r=0.688, p=0.001 and r=0.395,p=0.005, respectively). Moreover, the serum levels of HDL cholesterol levels were positively associated with leptin levels (r=0.350, p=0.013). Also, serum hemoglobin levels before IVIG treatment were positively correlated with leptin levels (r=0.356, p=0.011) (Table 6).
Discussion
We investigated the iron status in patients with KD to determine the role of hepcidin and ID parameters on the clinical outcome of KD. In the present study, serum hepcidin levels peaked after IVIG treatment in patients with KD and subsequently decreased in the convaslescent phase. In addition, IDA was more prevalent in patients of acute phase of KD compared with the control groups, even though the presence of iron deficiencyin the acute phase of KD did not indicate the poor outcomes such as CAL or IVIG resistance. Moreover, serum hepcidin levels were highest in the subacute phase of KD while iron and transferrin saturations were lowest in the acute phase.
| Parameters | CAL(+)(n = 11) | CAL(-)(n = 39) | P-value |
|---|---|---|---|
| Gender (male/female) | 9/2 | 15/24 | 0.011 |
| BMI, kg/m2 | 17.94 ± 1.71 | 16.61 ± 1.89 | 0.043 |
| Hemoglobin, g/dL | 11.33 ± 1.25 | 11.38± 0.86 | 0.867 |
| Neutrophil, % | 67.05 ± 13.09 | 63.47 ± 13.96 | 0.451 |
| CRP, mg/dL | 8.05 (1.0−17.49) | 5.57(0.78−18.45) | 0.566 |
| NT-proBNP, pg/mL | 833.94 ± 770.48 | 705.45 ± 797.17 | 0.637 |
| Albumin, g/dL | 4.00 ± 0.40 | 4.11 ± 0.30 | 0.318 |
| Iron, μg/dL | 16.82 ± 7.51 | 20.24 ± 18.98 | 0.566 |
| Transferrin saturation, % | 6.56 ± 2.43 | 8.18 ± 6.71 | 0.443 |
| Ferritin, μg/L | 152.35 ± 71.75 | 188.46 ± 98.64 | 0.270 |
| Hepcidin, ng/mL | 1.17 ± 0.13 | 1.60 ± 1.53 | 0.363 |
| TNF-α, pg/mL | 1.18 ± 3.71 | 0.11 ± 0.14 | 0.361 |
| IL-6, pg/mL | 522.41 ± 1580.62 | 125.69 ± 459.39 | 0.429 |
Table 4: : Relationship between clinical parameters in KD patients and development of coronary artery lesion in the acute phase.
| CAL | Acute phase | Convalescent phase | P-value |
|---|---|---|---|
| CA dilatation | 11 (20.0 %) | 2 (4.0%) | 0.004 |
| CA aneurysm | 0 | 0 | NA |
| LV fractional shortening (%) | 33.64 ± 4.98 | 35.27 ± 3.80 | 0.064 |
| E/A ratio | 1.36 ± 0.24 | 1.40 ± 0.16 | 0.252 |
| E/e’ ratio | 10.51 ± 2.03 | 9.27 ± 1.55 | 0.001 |
Table 5: : Cardiovascular findings on echocardiographic examination in 50 KD patients.
KD is an acute-onset systemic vasculitis of a composite of infection, genetic predisposition and marked activation of immune system [13]. During the acute phase of KD, monocytes, T cells and B cells were activated and proinflammatory cytokines such as IL-1, IL-2, IL-6, and TNF-α were elevated. Anemia is a frequent finding in patients with acute phase KD and is characterized with normocytic and normochromic anemia or slightly microcytic and hypochromic anemia. In previous reviews, the prevalence of anemia in patients with KD was up to 51.5% [14]. However, the incidence and clinical impact of IDA on coronary outcome in patients with KD has not been well studied.
IDA is the most common cause of anemia in small infants and children. It is often challenging to diagnose IDA especially in hospitalized young children due to coexistent acute infection. In this study, 34% of patients KD showed the laboratory findings compatible of IDA in the acute phase. On the contrary, IDA was found in 21.1% of febrile controls and 11.1% of afebrile controls. In the literature, lower levels of CD4+ T-lymphocytes in children with IDA contribute to the decreased cell mediated immunity, although IDA did not affect humoral mediated immunity [15]. The severity of anemia might affect the degree of immunologic response in patients with KD. At present, it is uncertain about the association between the states of ID and immnunologic susceptibility to KD.
Hepcidin is an iron regulating hormone and well-known biomarker for IDA. In anemia of inflammation, hepcidin blocks iron flows into plasma and regulates homeostasis of red blood cell [16]. Hepcidin levels decrease in IDA and increase in conditions with increased body iron [17]. Recent study found that urinary hepcidin is an important marker for diagnosing IDA rather than serum hepcidin [18]. In above-mentioned study, urinary hepcidin levels decreased as the severity of anemia increased. In our study, serum hepcidin assay was not useful for predicting coronary artery complications in patients with KD, although hepcidin levels were elevated in patients with KD compared with febrile control groups. Up to now, normal values of hepcidin were not validated. A previous study reported a wide variation in plasma hepcidin levels even among healthy children and showed a significant correlation between hepcidin levels and ferritin [19]. In this study, multiple regression analysis showed a negative correlation between serum hepcidin levels and TIBC levels.
In the literature, the increased hepcidin expression after IL-6 stimulation was found through a complex of the IL-6 receptor and gp130 dependent signaling cascades [20]. Therefore, we focused on the influences of hepcidin and interrelationship among different inflammatory cytokines in the present study. However, we didn’t find a direct relationship among circulating IL-6, TNF-α and hepcidin concentrations in patients with KD possibly due to the relatively small number of subjects. Also, serum hepcidin was not the prognostic factor for IVIG resistance or coronary artery complication in our patient cohort.
Iron deficiency can present with absolute form or overlapping feature with functional iron deficiency complicated by true deficiency of iron. The diagnosis of absolute iron deficiency in patients with KD is difficult because ID parameters such as ferritin and iron binding capacity are influenced by the presence of infection. The level of serum ferritin elevates in the presence of acute and chronic inflammation, malignancy, or liver disease, because it is an acute-phase reactant.Increased ferritin levels with decreased transferrin saturation and low TIBC indicates anemia of inflammation [21]. In the present series, serum ferritin levels were higher and TIBC was lower in patients with acute phase KD than those of the febrile controls.
It is not fully explained how long IDA persists in patients with KD. In patients with acute phase KD, transient hyposideremia was induced by inflammation-induced hepcidin [22]. Huang et al. has reported that hemoglobin levels increased at three weeks after IVIG treatment in patients with KD attributed to the time lag in erythropoiesis [23]. In the present study, 7 patients (14%) of KD showed persistent anemia in the convalescent phase, 2 patients of whom were IVIG resistant requiring second IVIG treatment. This indicates that severe inflammatory response in KD results in the prolonged anemia in patients with KD. We found 86% of patients with KD showed increased hemoglobin levels above the baseline in the convalescent phase without routine supplementation of iron. This results point to the relatively higher proportions of the functional IDA in the acute phase of KD due to the effect of inflammatory cytokines on iron metabolism. Therefore, we need to be careful to select the patients who require iron supplementation in patients with KD because both iron overload and deficiency can cause the negative effects on immune function. At present, the association between improvement in IDA or iron deficiency and clinical outcomes is unclear [24].
| Hepcidin | Leptin | IL-6 | ||||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| Age, mon | -0.085 | 0.556 | 0.553 | 0.001 | -0.062 | 0.667 |
| Weight, kg | -0.044 | 0.760 | 0.688 | 0.001 | -0.025 | 0.715 |
| BMI, kg/m² | -0.007 | 0.962 | 0.395 | 0.005 | -0.084 | 0.563 |
| Pre-IVIG hemoglobin, g/dL | 0.058 | 0.689 | 0.356 | 0.011 | -0.059 | 0.683 |
| Post-IVIG hemoglobin, g/dL | 0.135 | 0.351 | 0.123 | 0.396 | -0.006 | 0.970 |
| White blood cell, 10³/μL | 0.078 | 0.591 | 0.244 | 0.087 | -0.165 | 0.251 |
| CRP, mg/dL | 0.122 | 0.399 | 0.048 | 0.936 | 0.239 | 0.095 |
| Iron, μg/dL | 0.044 | 0.776 | 0.048 | 0.752 | -0.115 | 0.453 |
| TIBC, μg/dL | -0.336 | 0.024 | -0.094 | 0.537 | -0.205 | 0.178 |
| Ferritin, μg/L | 0.224 | 0.139 | 0.091 | 0.553 | -0.018 | 0.908 |
| HDL-C, mg/dL | -0.073 | 0.614 | 0.350 | 0.013 | -0.105 | 0.470 |
| NT-proBNP, pg/mL | 0.008 | 0.956 | -0.173 | 0.229 | 0.102 | 0.481 |
| Hepcidin, ng//mL | ― | ― | 0.142 | 0.325 | -0.086 | 0.551 |
| Leptin, pg/mL | 0.142 | 0.325 | ― | ― | -0.056 | 0.697 |
| TNF-α, pg/mL | -0.021 | 0.886 | -0.035 | 0.810 | -0.024 | 0.869 |
| IL-6, pg/mL | -0.086 | 0.551 | -0.056 | 0.697 | ― | ― |
Table 6: Correlation of pre-IVIG hepcidin, leptin and IL-6 with clinical and other laboratory variables in patients with Kawasaki disease.
Limitations
There were several limitations to our study. First, the time of blood sampling was not fixed in the patients. The levels of iron are higher more than twice in the morning than afternoon or evening due to daytime variations of serum iron [25]. Second, we did not routinely check the reticulocyte count, peripheral blood smear or Coombs’ test in patients with KD. Therefore, we could not rule out the possibility of hemolytic anemia after IVIG treatment. Hemolytic anemia after IVIG infusion could occur by the direct antibody mediated hemolysis of red blood cells [26]. Third, the afebrile control group is low in number. Lastly, there was no coronary artery aneurysm as a complication of KD in our patients.
Conclusion
In conclusion, we demonstrated the highest hepcidin concentrations with the lowest hemoglobin levels in the patients with subacute phase KD followed by subsequent decrease of hepcidin levels in the convalescent phase. IDA was more common in KD patients and elevated hepcidin levels were found in KD patients compared to febrile controls. However, the parameters of IDA and hepcidin levels did not predict IVIG resistance or coronary artery complications.Therefore, future research is needed to better clarify the role of hepcidin on the pathogenesis of systemic inflammation of KD and the coronary artery complications.
Ethical Aspect of Research
This study was approved by the Eulji University Hospital Institutional Review Board (No. 2017-07-018). Informed consent was obtained from the parents of all the children and was confirmed by the Eulji University Hospital Institutional Review Board.
Conflict of Interests
Authors have no financial or nonfinancial benefits have been received or will be received from any party related directly or indirectly to the subjects of this article. No potential conflicts of interest to this article was reported.
Acknowledgement
This research was supported by EMBRI Grants (2017 EMBRIDJ) from the Eulji University
Funding
Not applicable.
Newburger JW, Fulton DR. (2004) Kawasaki disease. Curr Opin Pediatr 6: 508-514.[ Ref ]
Hedrich CM, Schnabel A, Hospach T. (2018) Kawasaki disease. Front Pediatr 6: 198.[ Ref ]
Hara T, Nakashima Y, Sakai Y, Nishio H, Motomura Y, et al. (2016) Kawasaki disease: a matter of innate immunity. Clin Exp Immunol 186: 134-143.[ Ref ]
Li X, Chen Y, Tang Y, Ding Y, Xu Q, et al. (2018) Predictors of intravenous immunoglobulin - resistant Kawasaki disease in children: a metaanalysis of 4442 cases. Eur J Pediatr 177: 1279-1292.[ Ref ]
Nemeth E, Ganz T (2014) Anemia of inflammation. Hematol Oncol Clin North Am 28: 671-681.[ Ref ]
Atsumi Y, Sakakibara H, Morikawa Y, MiyataK, Yamagishi H, et al. (2020) Decreased hemoglobin after initial treatment is associated with treatment resistance in Kawasaki disease in Kobayashi risk stratification. World J Pediatr 16: 623-628.[ Ref ]
del Giudice EM, Santoro N, Amato A, Brienza C, Calabro P, et al. (2009) Hepcidin in obese children as a potential mediator of the association between obesity and iron deficiency. J Clin Endocrinol Metab 94: 5102-5107.[ Ref ]
Park CH, Valore EV, Waring AJ, Ganz T (2001) Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 276: 7806- 7810.[ Ref ]
Demirag MD, Haznedaroglu S, Sancak B, Konca C, Gulbahar O, et al. (2009) Circulating hepcidin in the crossroads of anemia and inflammation associated with rheumatoid arthritis. Intern Med 48: 421-426.[ Ref ]
.Kuo HC, Yang YL, Chuang JH, Tiao MM, Yu HR, et al. (2012) Inflammationinduced hepcidin is associated with the development of anemia and coronary artery lesions in Kawasaki disease. J Clin Immunol 32: 746- 752.[ Ref ]
McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, et al. (2017) Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation 136: e927-999.[ Ref ]
World Health Organization. (2001) Iron deficiency anemia: assessment, prevention, and control: a guide for program manager. Geneva: World Health Organization.[ Ref ]
Del Principe D, Pietraforte D, Gambardella L, Marchesi A, de Jacobis IT, et al. (2017) Pathogenetic determinations in Kawasaki disease: the haematological point of view. J Cell Mol Med. 21: 632-639.[ Ref ]
Gulhan B, Kesici S, Beken S, Cilsal E, Kale G, et. al. (2012) Varying clinical features of Turkish Kawasaki disease patients. Turk J Pediatr 54: 1-6[ Ref ]
Das I, Saha K, Mukhopadhyay D, Roy S, Raychaudhurl G, et al. (2014) Impact of iron deficiency anemia on cell-mediated and humoral immunity in children: A case control study. J Nat Sci Biol Med 5: 158-163.[ Ref ]
Mena NP, Esparza A, Tapia V, Valdes P, Nunez MT (2008) Hepcidin inhibits apical iron uptake in intestinal cells. Am J Physiol Gatrointest Liver Physiol 294: 192-198. [ Ref ]
Ganz T (2003) Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 102: 783-788.[ Ref ]
Dewan P, Dixit A, Gomber S, Kotru M, Banerjee BD, et al. (2019) Serum and urinary hepcidin for diagnosing iron deficiency anemia in under 5 children. J Pediatr Hematol Oncol 41: e216-e220.[ Ref ]
Kumar S, Bhatia P, Jain R, Bharti B (2019) Plasma hepcidin levels in healthy children: Review of current literature highlights limited studies. J Pediatr Hematol Oncol 41: 238-242.[ Ref ]
Pietrangelo A, Dierssen U, Valli K, Garuti C, Rump A, et al. (2007) STAT3 is required for IL-6-gp130-dependent activation of hepcidin in vivo. Gastroenterology 132: 294-300.[ Ref ]
Nairz M, Theurl I, Wolf D, Weiss G (2016) Iron deficiency or anemia of inflammation? : Differential diagnosis and mechanism of anemia of inflammation. Wien Med Wochenschr 166: 411-423.[ Ref ]
Huang YH, Kuo HC (2017) Anemia in Kawasaki disease: Hepcidin as a potential biomarker. Int J Mol Sci 18: 820.[ Ref ]
Huang YH, Huang FC, Yu HR, Hsieh KS, Yang YL, et al. (2016) Hepcidininduced iron deficiency is related to transient anemia and hypoferremia in Kawasaki disease patients. Int J Mol Sci 17: 715.[ Ref ]
McDonagh MS, Blazina I, Dana T, Cantor A, Bougatsos C (2021) Screening and routine supplementation for iron deficiency anemia: a systematic review. Pediatrics 135: 723-733.[ Ref ]
Dale JC, Burrritt MF, Zinsmeister AR (2002) Diurnal variation of serum iron, iron-binding capacity, transferrin saturation, and ferritin levels. Am J Clin Pathol 117: 802-808.[ Ref ]
Berrad R, Whittemore B, Scuccimarri R (2012) Hemolytic anemia following intravenous immunoglobulin therapy in patients treated for Kawasaki disease: a report of 4 cases. Pediatr Rheumatol Online J 10: 10.[ Ref ]