Journal Name: Journal of Pediatrics and Infants
Article Type:Research
Received date:15 July, 2021
Accepted date:26 August, 2021
Published date:02 September, 2021
Citation:Fejes M, Varga B, Molnar D, Hollody K (2021) Factors affecting the Body Composition in Childhood Epilepsy. J Pediat Infants Vol: 4, Issu: 2 (32-39).
Copyright:© 2021 Fejes M et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: The aim of our present study was to investigate the effect of epilepsy, antiepileptic drug treatment and sociodemographic factors on the nutritional status of children and adolescents with epilepsy
Purpose: This was a clinical, case-control study. Patients with epilepsy (139)were comparedwith children from the general population (GP) (232).
Results: Three BMI classifications were used: Hungarian, WHO and IOTF. More very thin children were found in both epilepsy and general population groups. The most children with epilepsy had normal body weight. The age, sex and duration of epilepsy did not influence special features of epilepsy. The proportion of overweight children was higher on polytherapy, while children on monotherapy were rather normal or thin. The epilepsy syndromes had mainly normal physique, idiopathic localization related epilepsy had more overweight patients.
Medium education and inactive status in the labour market occur at a much higher rate of underweight. Higher quality schools or catching up class tend to cause weight loss or obesity. The only child with epilepsy in a family was mostly obese.
Most, of the differences considering HRQoL domains occurred both in children with epilepsy and GP in those who had normal body shape.
Conclusion:It is clear from our data that the body composition of children with epilepsy was at least as dependent on environmental factors as the forms of epilepsy, and it was not clear whether medication had changed it. The nutritional status of children with epilepsy can be considered multifactorial.
Keywords: Epilepsy, BMI, Nutrition aspect, Quality of life, Children, Adolescents.
Introduction
In welfare societies rapid economic, social, nutritional and lifestyle changes have emerged in recent decades. Worldwide obesity has nearly tripled since 1975 [1]. The development of obesity has multifactorial origin and is influenced by several genetic, environmental and sociocultural factors. The relatively cheap fast foods, the incresaed screen time and consequential sedentary lifestyle all grow the risk of obesity and probably have contributed to the rapid rise of obesity not only in adults but in children and adolescents as well. Early obesity influencess the development of many civilization diseases and it affects not only the physical, but also mental and social well-being of the individual as well. In the long run it increases health costs, worsens life prospects and has a significant economic impact [2]. Undernutrition and overweight frequently occur within the same family of GP and E [3]. The global prevalence of underweight (thinness) among children and adolescents – defined as less than 2 SDs from the median for body mass index (BMI) by age and sex – is 8.4% for girls and 12.4% in boys [4]. The modern ideal thin body shape conveyed by social media can contort the body image of young people [5]. Body image dissatisfaction among adolescent girls was found high despite four in five girls had normal weight [6]. Undernutrition may occur in patients with chronic diseases. Considering epilepsy most of the published studies reported obesity as consequence. Ladino et al reported that children with early onset epilepsy or with idiopathic epilepsy syndromes are more prone to obesity and they suspected that obesity can be related to an underlying genetic abnormality [7]. De Azevedo Fernandez et al found that calorie intake was higher and the quality of food composition was lower than the recommended values in patients with epilepsy [8]. The majority of children with epilepsy are overprotected and do not perform regular physical activity. Some antiepileptic drugs (AEDs) can cause higher appetite or lethargy, depression, anxiety, decreased physical activity and later as a result overweight.
The number of reports about the nutritional status of patients with epilepsy is relatively high and most of them emphasize higher obesity rate with these people [8-10]. Less data are available on the nutritional status of children with epilepsy, and studies about their undernutrition are scarce. The aim of our present study was to investigate the effect of epilepsy, antiepileptic drug treatment and sociodemographic factors on the nutritional status of children and adolescents with epilepsy.
Methods
Clinical, non-interventional case-control study was designed
Patients with epilepsy:139 children aged 8-18 years with epilepsy were prospectively recruited in two regional paediatric medical centres: Department of Paediatrics of the University of Pécs and the Borsod County Central Hospital [12-14]. All children with epilepsy were regularly followed-up by one of the paediatric neurologist authors (M.F. and K.H.) The main characteristics of their epilepsy and antiepileptic drug treatment were collected from the clinical/hospital databases. Classification of their epileptic seizures/syndromes were done according to the ILAE criteria [15,16]. Duration of their epilepsy was recorded in years. The following four categories of seizure frequency were used: least frequent (maximum 1/year or seizure free), less frequent (1 or 2 per month), more frequent (≥3 per week), and most frequent (daily). Epileptic children were also groupt according tot he number of drugs taken simultaneously (1 drug=monotherapy; 2-3 antiepileptic drugs=polytherapy). Neither of patients with epilepsy were on ketogenic diet nor had epilepsy surgery.
Control population: 232 children (8-18 years old) made up the general population (GP). They were recruited from the elementary and secondary schools of the above mentioned two regions 71 children /28.08% were from the South-West region and 161 children/ 71.98 % were from the North-East region of Hungary. All children lived without any chronic disease.
Two age groups were created as follows: 8-12 and 13-18 years. Throughout this study, when children and adolescents were considered together, they were collectively called ’children’.
Body weight and height of the children were measured at the neurological follow-up visit or at the school’s doctor office by trained assistants. Body mass index (BMI) was calculated from the body weight and height data.
Hungarian standards were used for the evaluation of the nutritional status of patients studied [11]. Five BMI categories were differentiated: very thin (< 3 percentile), thin (<10 and ≥3 percentile), normal (N, ≥10 and <90 percentile), overweight (OW≥90 and <97 percentile), obese (O >97 percentile). BMI categories were compared not only with the Hungarian, but also with the international standards as well (extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity and WHO cut-offs) [12,13].
Health Related Quality of Life (HRQoL) of both populations was measured by the KIDSCREEN-52 questionnaire [14]. Children were asked to fill in the questionnaire after the follow-up visits or at the schools. Patients with severe motor or intellectual disabilities were not involved. Children with mild mental retardation were not excluded, but it was essential that the child could understand the questions and answer them clearlySocial demographic data were collected about education level and occupation of parents, family relationship of the parents and about sibling/s. Parents were divided in three groups according to their education levels (1/ ≤ 8 primary classes, 2/ secondary and 3/ high education). Active workers and non-working parents were classified. Considering family relationships, we created two groups: cohabiting or single parents.
Data were collected between November 2012 and February 2016.
Statistical analysis: Statistical analysis was performed using IBM SPSS 24 software. Levene’s test was performed to assume equal variances or homogeneity of variance whenever independent samples t-test was necessary. T-test was used to determine significance and difference between the means. The confidence interval (CI) was 95% and statistical significance was set at p <0.05. The strength of correlation (r) was: weak=0-0.25, medium weak = 0.25-0.5, medium strong = 0.50-0.75, or strong = 0.75-1. Parametric and non-parametric statistic probes were used for analysis (ANOVA, paired sampled t-tests and F-tests, Pearson Chisquared probe, Kruskal- Wallis test), which showed whether a significant correlation existed between the variables.
Linear regression was performed to measure possibility of linear relationship. The number of antiepileptic drugs, seizure frequency, and duration of epilepsy were correlated to BMI. The range of R-squared values was 0 to 1. The beta coefficient (β1) is the degree of change in the outcome variable for every 1-unit of change in the predictor variable. The beta coefficients can be negative or positive.
Risk rates (RR) were calculated relative to the GP’ BMI data for each BMI category of epileptic patients and for each variable (RR: CIE /CIGP ; CI: Cumulative incidence).
For measuring effect sizes (ES), Cohen’s d was used, which defines the difference between two means divided by the standard deviation for the data. ES values were very small d=0.01; small 0.2; medium 0.5; large 0.8; very large 1.2 and huge 2.0.
Results
Descriptive information of the children with epilepsy and the general population
139 families with epileptic child were recruited. Their mean age was 15 years and 7 months, 80 boys (55.6 %) and 64 girls (44.4 %).
According to the ILAE epilepsy classification: 34 (24.46%) had idiopathic localization-related epilepsy (ILRE), 64 (46.04%) idiopathic generalized epilepsy (IGE), 4 (2.87%) symptomatic generalized epilepsy (SGE) and 33 (23.74 %) symptomatic focal epilepsy (SPE). 4 (2.87%) had non classificable E. Mean duration of their epilepsy was 4.3 years (SD 2.92 years). 96 (66.7%) patients were on monotherapy, and 39 (27.1 %) on polytherapy. 4 patients were on drug withdrawal.
232 families formed the general population. The children’s mean age was 12 years 11 months (SD 7 months) 114 (49.14 %) boys and 118 (50.86 %) girls.
Physical parameters
The height distribution curve corresponded to the normal distribution either children with epilepsy or GP (Table 1). Boys with epilepsy had higher height, but significant difference was not found (Table 2).
The median values of body weight distribution were nearer to the slim values in both groups, but the proportion of thin children was higher in the GP’ group. The rate of obesity or overweight did not exceed the statistically expected values (Table 3). Taking into account age and gender, boys over 13 years of age weighed more than the girls, but no significant difference was measurable (Table 2).
Nutritional status (BMI values) of children with epilepsy (E) and from the general population (GP) was investigated according to the three classification systems (Hungarian, WHO and IOTF). The significances were higher in the thin groups as there were a higher proportion of thin and skinny children in the GP group. Children with epilepsy were more likely to have a normal physique, and there was no higher proportion of overweight obese patients. In normal, overweight, obese categories of epileptic patients, the mean BMI is higher within a category, but a significant difference can only be measured in the normal BMI group. The IOTF system indicates a higher proportion of predominance than the other two scales. There is no significant difference between the three classification systems (p: 0.26-0.50) (Table 3).
| Physical parameters | Levene | t-test | |||||
|---|---|---|---|---|---|---|---|
| Height (cm) | Mean ±SD | F | p | t-value | df | p | st error |
| Total E | 157,63±16,45 | 2,002 | 0,158 | -1,475 | 364 | 0,141 | 1,411 |
| GP | 155,14±15,04 | 0,992 | |||||
| <13 year E | 143,13±13,61 | 5,275 | 0,023 | 0,571 | 159 | 0,569 | 1,87 |
| <13 year GP | 144,22±10,11 | 0,973 | |||||
| 13-18 years E | 168,8±10,29 | 1,346 | 0,247 | -1,308 | 203 | 0,192 | 1,129 |
| 13-18 years GP | 164,8±11,71 | 1,06 | |||||
| Weight (kg) | |||||||
| Total E | 49,24±20,85 | 7,659 | 0,006 | -2,138 | 380 | 0,033 | 1,738 |
| GP | 45,07±16,93 | 1,097 | |||||
| <3 year E | 36,31±16,59 | 2,56 | 0,111 | 0,048 | 170 | 0,961 | 2,159 |
| <13 year GP | 36,42±12,90 | 1,213 | |||||
| 13-18 years E | 58,22±18,74 | 1,833 | 0,177 | -2,187 | 208 | 0,03 | 2,032 |
| 13-18 years GP | 52,89±16,36 | 1,463 | |||||
| BMI | |||||||
| Total E | 20,13±4,56 | 1,143 | 0,286 | -0,619 | 277 | 0,536 | 0,755 |
| GP | 18,88±3,49 | 0,259 | |||||
| <13 year E | 18,05±4,42 | 0,22 | 0,64 | 0,404 | 159 | 0,687 | 0,608 |
| <13 year GP | 17,76±4,27 | 0,411 | |||||
| 13-18 years E | 21,47±4,14 | 5,913 | 0,016 | -3,017 | 201 | 0,003 | 0,458 |
| 13-18 years GP | 19,89±3,29 | 0,299 | |||||
Table 1: Physical parameters of GP and E patients and their difference(no)
| Covariance analysis | |||||||
|---|---|---|---|---|---|---|---|
| N | Mean | SD | F | p | |||
| <13 years | BMI | Boys | 12 | 16.64 | 3.11 | 0.39 | 0.85 |
| Girls | 16 | 16.87 | 3.12 | ||||
| Total | 28 | 16.73 | 3.05 | ||||
| Height | Boys | 12 | 136.81 | 12.03 | 0.27 | 0.87 | |
| Girls | 16 | 136.08 | 10.78 | ||||
| Total | 28 | 136.50 | 11.31 | ||||
| Weight | Boys | 12 | 32.00 | 11.45 | 0.007 | 0.93 | |
| Girls | 16 | 31.67 | 8.98 | ||||
| Total | 28 | 31.86 | 10.28 | ||||
| >13 years | BMI | Boys | 51 | 21.07 | 4.3 | 0.16 | 0.69 |
| Girls | 60 | 20.74 | 4.64 | ||||
| Total | 111 | 20.92 | 4.45 | ||||
| Height | Boys | 51 | 163.45 | 15.1 | 0.32 | 0.58 | |
| Girls | 60 | 162.08 | 9.42 | ||||
| Total | 111 | 162.82 | 12.79 | ||||
| Weight | Boys | 51 | 57.65 | 18.51 | 0.72 | 0.39 | |
| Girls | 60 | 54.94 | 14.39 | ||||
| Total | 111 | 56.41 | 16.73 | ||||
Table 2: BMI, height and weight and their relationships to the age and sex by covariance analysis in children with epilepsy. (BMI: Body Mass Index, N=number, SD=standard deviation, F= F-test)
| Classification | Value | Epilepsy | General Population | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hungarian | Percentile | No | % | Mean | SD | No | % | Mean | SD | P | RR |
| Very thin | <P3 | 5 | 3.47 | 14.24 | 1.84 | 24.00 | 10.13 | 15.01 | 2.18 | 0.006 | 0.34 |
| Thin | <P10 and ≥P3 | 12 | 8.33 | 16.12 | 2.43 | 26.00 | 11.81 | 16.04 | 2.14 | 0.001 | 0.71 |
| Normal | ≥P10 and <P90 | 102 | 70.83 | 19.23 | 8.34 | 146.00 | 60.76 | 18.72 | 5.21 | <0.001 | 1.17 |
| Overweight | ≥P90 and ≤P97 | 10 | 6.94 | 25.84 | 3.89 | 18.00 | 7.59 | 23.28 | 4.11 | 0.48 | 0.91 |
| Obese | >97 | 10 | 6.94 | 30.57 | 4.42 | 18.00 | 7.59 | 26.35 | 5.59 | 0.018 | 0.91 |
| WHO | Standard deviation | ||||||||||
| Very thin | <- 2 SD | 5 | 3.47 | 14.37 | 1.53 | 17.00 | 7.17 | 15.30 | 2.07 | 0.002 | 0.48 |
| Thin | <-1SD and ≥- 2 SD | 12 | 8.33 | 16.07 | 2.74 | 31.00 | 13.80 | 15.85 | 1.93 | 0.004 | 0.64 |
| Normal | ≥-1and ≤1SD | 101 | 70.14 | 19.17 | 8.13 | 146.00 | 60.60 | 18.67 | 5.20 | <0.001 | 1.14 |
| Overweight | >1SD and ≤2 SD | 9 | 6.25 | 25.97 | 4.03 | 19.00 | 8.02 | 23.24 | 3.92 | 0.38 | 0.78 |
| Obese | >2SD | 11 | 7.64 | 30.11 | 6.31 | 18.00 | 7.59 | 26.35 | 5.59 | 0.076 | 1.01 |
| IOTF | BMI at 18 years | ||||||||||
| Very thin | <17 | 4 | 2.78 | 13.64 | 0.81 | 17.00 | 7.17 | 15.03 | 1.22 | 0.001 | 0.39 |
| Thin | <18,5 and ≥17 | 14 | 8.33 | 16.35 | 2.37 | 32.00 | 13.50 | 15.96 | 2.12 | 0.002 | 0.72 |
| Normal | 18,5 and <25 | 99 | 68.75 | 19.14 | 8.12 | 144.00 | 60.76 | 18.68 | 5.10 | <0.001 | 1.13 |
| Overweight | 25 and <30 | 11 | 7.64 | 25.50 | 4.58 | 20.00 | 8.44 | 23.17 | 3.80 | 0.67 | 0.91 |
| Obese | ≥30 | 11 | 7.64 | 26.31 | 6.31 | 18.00 | 7.59 | 26.35 | 5.59 | 0.76 | 1.01 |
| Abbreviations: BMI: Body Mass Index, E: epilepsy, GP: general population, RR: risk rate, P: percentile, SD: standard deviation, WHO: World Health Organisation, IOTF: International Obesity Task Force. | |||||||||||
Table 3: Nutritional status of children with epilepsy (E) and from the general population (GP) according to the three classification systems (Hungarian, WHO and IOTF) using by BMI values. There is no significant difference between the three classification systems ( p: 0.26-0.50)
| Total number | Very thin | Thin | Normal | Overweight | Obese | |
|---|---|---|---|---|---|---|
| Epilepsy syndromes | ||||||
| ILRE | 34 | 0,00 | 0,48 | 1,17 | 1,52 | 1,14 |
| IGE | 63 | 0,31 | 0,66 | 1,18 | 1,04 | 1,04 |
| SGE | 3 | 0,00 | 0,00 | 1,64 | 0,00 | 0,00 |
| SFE | 30 | 0,61 | 1,03 | 1,04 | 0,40 | 0,81 |
| Antiepileptic drug therapy | ||||||
| Monotherapy | 96 | 1,15 | 0,00 | 1,09 | 0,56 | 0,97 |
| Polytherapy | 40 | 0,75 | 1,06 | 0,94 | 1,33 | 0,67 |
| Valproate | 60 | 0.50 | 0.83 | 1.06 | 1,28 | 0.84 |
| Valproate monotherapy | 46 | 0.43 | 0.72 | 1.11 | 1.10 | 1.10 |
| Carbamazepine | 25 | 0.1 | 0.66 | 1.09 | 1.53 | 0.51 |
| Carbamazepine mono. | 19 | 0.5 | 0.42 | 1.27 | 0.06 | 0.64 |
| Controlled E | 130 | 0,23 | 0,72 | 1,17 | 0,92 | 1,03 |
| Parents' Education | ||||||
| ≤8 classes | 18 | 0,33 | 0,67 | 1,60 | 0,67 | 0,44 |
| Medium | 85 | 0,28 | 1,25 | 1,07 | 0,88 | 1,21 |
| High graduated | 32 | 0,47 | 0,40 | 1,29 | 0,00 | 0,00 |
| Parents' Occupation | ||||||
| Active | 95 | 0,20 | 0,58 | 1,23 | 0,64 | 0,96 |
| Inactive | 36 | 0,75 | 2,50 | 0,92 | 0,67 | 0,67 |
| Family Relationship | ||||||
| Living Together | 107 | 0,24 | 0,66 | 1,16 | 0,80 | 1,06 |
| Single parent | 25 | 1,12 | 1,12 | 1,06 | 2,24 | 0,28 |
| Type of School of the children | ||||||
| Normal | 106 | 0,27 | 0,66 | 1,19 | 0,73 | 0,94 |
| Special | 26 | 0,66 | 1,65 | 0,92 | 1,65 | 0,33 |
| Siblings | ||||||
| None | 33 | 0,00 | 0,00 | 1,01 | 1,39 | 1,67 |
| 1 sibling | 58 | 0,29 | 0,58 | 1,35 | 0,50 | 0,39 |
| ≥2 siblings | 53 | 0,61 | 1,12 | 0,98 | 0,86 | 1,15 |
| Abbreviations: E: epilepsy. GP: general population; y: Year. BMI: body mass index. ILRE: Idiopathic localization-related epilepsy. IGE: Idiopathic generalized epilepsy. SFE: Symptomatic focal epilepsy. CI: cumulative incidence. RR:risk ratio. | ||||||
Table 4: Risk rate of variables and sociodemographic characteristics of epilepsy relative to the general population based on CIE and CIGP
Special features of epilepsy
Epilepsy syndromes: more children with idiopathic localization related epilepsies (ILRE) were overweight (RR: 1.52) and less children with idiopathic generalized epilepsy syndromes (IGE) were thin or very thin. (RR: 0.31- 0.66) while patients with symptomatic focal epilepsy (SFE) were mainly thin and normal (12% and 62 %). But it must be mentioned that the number of SGE patients was very low (Table 4).
No statistical difference was found considering duration time of epilepsy and seizure frequency on the basis of the age and gender.
BMI values of patients were not significantly influenced by the duration of epilepsy using linear regression analysis (E: β1:0.08; R2:0.02; r: 0.13).
Medium weak correlation was established between seizure frequency and duration time of epilepsy (r: 0.352, p=0.000) and no correlation was found between seizure frequency and BMI (β1:0.01; R2:0.003; r: 0.16).
Children on monotherapy were found to be mostly very thin (RR: 1.15). Valproate and carbamazepine were the most frequently used drugs in case of monotherapy. Nor valproate, nor carbamazepine had caused notable weight gain (Table 4). Those on polytherapy were frequently overweight (RR: 1.33). In the well-controlled epilepsy group, children were mainly normal-built. (RR:1.17) (Table 4).
Social demographic data
The children of parents with secondary levels of education turned out to be more obese and thinner than off springs of the highly educated parents.
Children of non-working parents were thinner, than offspring of the active workers.
The children with epilepsies in single parent families were more overweight, than in the “double” parent families. (RR:2.24)
Those children who attended special schools with high level qualification were more thinner and overweight, than the normal schools (RR:1.65). Families with only child showed the highest rate of overweight and obesity. On the contrary, in families with at least 3 children more siblings were thin. Among the siblings there were only 11 children with epilepsy, some of them were thin or obese.
No remarkable difference was observed between the North-East and South-West regions considering the BMI values of children.
Health Related Quality of Life of children with epilepsy compared to the general (control) population
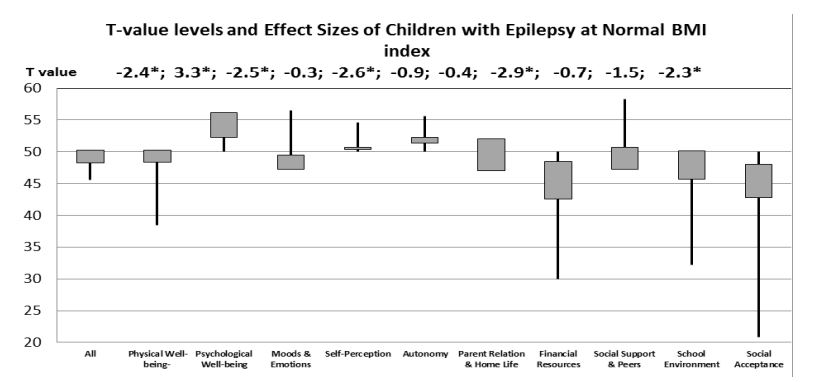
No statistically significant difference was found in the HRQoL of children with epilepsy and of the general population. Obese, normal and very thin children with epilepsy assessed their HRQoL poorer in the domains of emotional life and financial resources. Most of the differences considering HRQoL domains occured both in children with epilepsy and GP in those who had normal body shape. The scores of physical quality of life with children with epilepsy showed lower values than GP in normal body shape (Figure 1).
Figure 1:T value levels of different HRQoL domains of normal BMI children with E related to GP population with normal BMI (GP mean= 50. SD=10) on the block-spot graph. The mean value of GP is taken as 50 and the standard deviation is 10. Above the graph are shown the corresponding values of effect sizes. Significance levels *p ≤ 0.05.
Discussion
The essential aim of our study was to measure and compare the nutritional status of children with and without epilepsy and to investigate the relationship between epilepsy disease and nutritional status. As far as we know, there has been no similar study have also been published about children and adolescents with epilepsy up to now.
Excess weight gain did not develop in patients with wellcontrolled epilepsies and on monotherapy. The proportion of overweight was higher in children on polytherapy. In the case of valproate and carbamazepine monotherapy normal BMI’s occurs more frequently. Our patients with ILRE were more overweight, than those with SFE.
We also found that not only the epilepsy itself, but also familiar environment (parents’ education, employment, relationship and number of siblings) influences the body shape of the children as well. Slim, lean physique was high in our patients and the children of the general control population. There are no more obese/overweight children with epilepsy in the normal schools compared to the GP. Higher quality schools or catching up class tend to develop eating disorders that may rather cause weight loss or overweight. It is also a notable observation from our data that the only child with epilepsy in a family is mostly obese, while those children who live together with healthy or epileptic siblings are rather normal or thin.
We further found that health related quality of life of children was influenced by body composition. Therefore, it is worthwhile to measure disease-specific differences with a normal physique.
Ladino reported that obesity and overweight were found more frequently in patients with generalized epilepsy and idiopathic syndromes [7]. The majority of their overweight or obese patients with epilepsy had localization-related type of epilepsy as Janousek et al. studied [10].
Many papers reported weight gain and development of metabolic syndrome in patients with epilepsy [9,17-19]. In contrast, Caksen et al found that long-term use of valproate had not caused weight gain in pre pubertal children [20]. Sharpe et al. found that patients in childhood were less prone to weight gain on valproate therapy [21]. Changes in weight Z-score and BMI were significantly correlated with the initial weight Z-score and initial BMI on valproate therapy [22]. Weight gain, metabolic syndrome were most commonly reported on valproate treatment as side effect of therapy and weight gain was influenced by the dose of valproate [9].
Ladino el al did not observe correlation between obesity and drug-resistant epilepsy, while Janousek at al. found that patients with drug-resistant epilepsy and on polytherapy were mostly overweight and obese [7,10]. In Gao et al’ study seizure frequency was higher in the underweight and extreme obese adult populations, than in patients with normal BMI [23].
Researchers from Brazil reported that adults with epilepsy had had 2-4 times higher carbohydrate and protein intake than recommended.8 The nutritional behavior of adolescents, especially of those with epilepsy is inadequate. They have an increased risk of eating disorder; consequently they are less satisfied with their own appearance [24].
Wong et al reported that physical activity of teens with epilepsy is reduced compared to their siblings without epilepsy, and their BMI is higher in the adolescent age group [25].
The most differences HRQoL dimensions were measured among children with normal physique are epileptic or not. Probably in these cases the body composition did not affect their quality of life and we were able to measure the diseasespecific values.
Similar to Rani et al, we found that being bullyed (mainly in school) for being epileptic worsens the social acceptance of children [26].
Stress level is usually higher in parents with lower/ less education, lower socioeconomic status or/and lower incomes, which can influence not only the parents’ quality of life negatively but consequently the HRQoL of children with epilepsy as well. Perceived stigma occurs at a significantly higher rate in children with epilepsy and it negatively affects the life of the child and, can also cause behavioral problems.26 Socioeconomic deprivation also increases the risk of behavioral problems in children with epilepsy [27]. The rate of seizures in the population aged 0-18 is increasing with the deterioration of the socio-economic status [28].
Why is it widely believed, even by neurologists, that patients with epilepsies are gaining weight? One of the reasons that the doctor may see a well-balanced, seizure-free patient only when prescribing a medication is recommended, while a patient who is difficult to treat and takes more antiepileptic drugs is much more likely to be recalled back for control investigation.
Several aspects have to be considered when selecting an antiepileptic drug therapy, apart from type of seizures and epilepsy syndrome the age, the gender, the cognitive status, the behavior of the child should also be considered, along with the possible side effects of the antiepileptic drug that affects the body weight. The optimal antiepileptic drug choice would be the so-called weight-neutral medication. Not only the child with epilepsy but his/her family members have to be educated how they can avoid the possible side effects of the prescribed medicine to maintain healthy nutrition. It is still common in schools that children with a medical diagnosis of epilepsy are not allowed to take part in the regular physical education classes regardless of what type of seizures or epilepsy syndrome they have. Teachers need to be made aware that the majority of children with well-controlled epilepsy can do physical activities. Some children with epilepsy need psychological support (intensive behavior therapy).
Our study has several strengths. It included a relatively large patient group with epilepsy and regional, age matched, healthy control cohort for comparison as well. Our data showed the effect of epilepsy disease and environmental factors on BMI in a novel form. We also assessed not only the somatic, but also other factors, more precisely the quality of life of the children and adolescents with and without epilepsy, how their body shapes influence their quality of life.
Weaknesses are that we had not obtained precise data about calorie intake and physical activity and the number of patients with different epilepsy syndromes was limited to draw firm conclusions.
Conclusion
In our study, we found that children with epilepsy are rather normal-built than obese. It is clear from our data that the body composition of children with epilepsy was at least as dependent on environmental factors as the forms of epilepsy, and it was not clear whether medication had changed it. The nutritional status of children with epilepsy can be considered multifactorial.
Declarations
Disclosures and funding
The authors have stated that they had no interests which might be perceived as posing a conflict or bias.
Source of funding
The authors and their institutions have not received payment or services from a third party (government, commercial sources, private foundation, etc.) for any aspects of the submitted work (including but not limited to grants, data monitoring board, study design, manuscript preparation, statistical analysis, etc.) at any time.
Ethics approval
Approval was obtained from the Regional Science Ethical Committee of the University of Pécs and from the Regional/ Local Committee of Science and Research Ethics of BorsodAbaúj-Zemplén, Heves and Nógrád Counties. Informed consent was obtained from the children and their parents.
Source of funding: The authors and their institutions have not received payment or services from a third party (government, commercial sources, private foundation, etc.) for any aspects of the submitted work (including but not limited to grants, data monitoring board, study design, manuscript preparation, statistical analysis, etc.) at any time.
Authors’ contribution
M.F. had the original idea. K.H., M.F. collected the data. Data entry was done by M.F. and B.V. The statistical analysis was carried out by M.F. and B.V. Major revision was taken by D.M. All authors contributed to data interpretation and writing the manuscript. All authors read and approved the final manuscript. A native English reviewer checked the grammar and the composition.
Acknowledgement
Authors would like to thank the participating families with epileptic and health children. Big thank goes to Antal Furedi for programming support. Jozsef Teglas helped dates’ input. F.M. would like to thank to the management of Borsod County Hospital: dr. Gabor Csiba, prof. dr.Laszko Barkay, prof. dr. Kalman Nagy for permission of using intranet and other databases. Thank you very much for the support provided by dr. Imre Velkey, regional chief childneurologist and their assistants.
WHO (2018) Obesity and overweight. [ Ref ]
McGrowder DA (2018) Biochemical parameters as cardiovascular risk factors in obese children and adults. J Endocrinol Diabetes Obes 6: 1115. [ Ref ]
WHO (2019) Malnutrition [ Ref ]
Christian P, Smith ER (2018) Adolescent Undernutrition: Global Burden, Physiology, and Nutritional Risks. Ann Nutr Metab 72: 316-328. [ Ref ]
Jordan AB, Kramer-Golinkoff EK, Strasburger VC (2008) Does Adolescent Media Use Cause Obesity and Eating Disorders? Adolesc Med 19: 431-449. [ Ref ]
Pokhrel S, Acharya B, Adhikari C (2015) Nutritional Status and Body Image Dissatisfaction among Adolescent Girls in Kaski District, Nepal. International Journal of Health Sciences & Research 5: 462-469. [ Ref ]
Ladino LD, Hernández-Ronquillo L, Téllez-Zenteno JF (2014) Obesity and its association with generalised epilepsy, idiopathic syndrome, and family history of epilepsy. Epileptic Disord. 16: 343-353. doi: 10.1684/ epd.2014.0677. [ Ref ]
. de Azevedo Fernandez R, Corrêa C, Muxfeld Bianchim M, Schweigert Perry ID (2015) Anthropometric profile and nutritional intake in patients with epilepsy. Nutr Hosp 32: 817-822. 9. Fang J, Chen S, Tong N, Chen L, An D, et al. (2012) Metabolic syndrome among Chinese obese patients with epilepsy on sodium valproate. Seizure. 21: 578-582. [ Ref ]
Janousek J, Barber A, Goldman L, Klein P (2013) Obesity in adults with epilepsy. Epilepsy Behav 28: 391-394. [ Ref ]
Ágfalvi R, Blatniczky L, Darvay S, Joubert K (2004) [Guidelines and tables for assessing childhood nutrition ] National Institute of Child Health. [ Ref ]
Cole TJ, Lobstein T.(2012) Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes 7: 284-294. [ Ref ]
World Health Organization (2020) Comparison with IOTF cut-offs (boys). [ Ref ]
The KIDSCREEN Group Europe (2006) The KIDSCREEN Questionnaires - Quality of life questionnaires for children and adolescents. Pabst Science Publishers, Lengerich. [ Ref ]
Rudzinski LA, Shih JJ (2011) The Classification of Seizures and Epilepsy Syndromes. Novel Aspects on Epilepsy. InTech Open. [ Ref ]
Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, et al. (2014) A practical clinical definition of epilepsy. Epilepsia 55: 475-482. [ Ref ]
Pachachi OA, Thanoon IA (2011) Body mass index and some biochemical parameters among valproate treated male epileptic patients. Ann Coll Med Mosul 37: 114-121. [ Ref ]
Abaci A, Saygi M, Yis U, Demir K, Dirik E, et al. (2009) Metabolic alterations during valproic acid treatment: a prospective study. Pediatr Neurol 41: 435-439. [ Ref ]
Carmona-Vazquez CR, Ruiz-Garcia M, Pena-Landin DM, Diaz-Garcia L, Greenawalt SR (2015) [The prevalence of obesity and metabolic syndrome in paediatric patients with epilepsy treated in monotherapy with valproic acid]. Revista de neurologia 61: 193-201. [ Ref ]
Caksen H, Deda G, Berberoğlu M (2002) Does long-term use of valproate cause weight gain in prepubertal epileptic children? Int J Neurosci 112: 1183-1189. [ Ref ]
Sharpe C, Wolfson T, Trauner DA (2009) Weight Gain in Children Treated With Valproate. J Child Neurol 24: 338-341. [ Ref ]
Novak GP, Maytal J, Alshansky A, Eviatar L, Sy-Kho R, et al. (1999) Risk of Excessive Weight Gain in Epileptic Children Treated with Valproate. J Child Neurol 14: 490-495. [ Ref ]
Gao S, Juhaeri J, Dai WS (2008) The incidence rate of seizures in relation to BMI in UK adults. Obesity (Silver Spring) 16: 2126-2132. [ Ref ]
Kolstad E, Bjork M, Gilhus NE, Alfstad K, Clench-Aas J,et al. (2017) Young people with epilepsy have an increased risk of eating disorder and poor quality diet. Epilepsia Open 3: 40-45. 25. Wong J, Wirrell E (2006) Physical activity in children/teens with epilepsy compared with that in their siblings without epilepsy. Epilepsia 47: 631-639.[ Ref ]
Rani A, Thomas PT.(2019) Stress and perceived stigma among parents of children with epilepsy. Neurol Sci 40: 1363-1370. [ Ref ]
Carson J, Weir A, Chin RF, McLellan A (2015) Socioeconomic deprivation is an independent risk factor for behavioural problems in children with epilepsy. Epilepsy Behav 45: 105-109. [ Ref ]
Potdar PS (2018) Study of some epidemiological aspects of seizure disorders among children in rural area of a district. IJCMPH 5: 2888- 2893. [ Ref ]