Journal Name: Journal of Pediatrics and Infants
Article Type: Short communication
Received date: 04 December, 2020
Accepted date: 25 January, 2021
Published date: 01 February, 2021
Citation: Shaik M, Wagh D, Athikarisamy S (2021) INSURE (INtubate, SURfactant and Extubate) Method in Preterm Infants with Respiratory Distress Syndrome: A Retrospective Study. J Pediat Infants Vol: 4, Issu: 1 (32-38).
Copyright: © 2021 Shaik M et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Preterm infants with severe respiratory distress syndrome (RDS) are usually managed with endotracheal intubation and surfactant administration followed by mechanical ventilation however this has immediate and long-term complications. Hence, INSURE (Intubate, surfactant administration and extubate) method combined with continuous positive airway pressure (CPAP) support has been accepted as an alternative method in eligible infants. Aim of this study is to evaluate the failure rate of INSURE method and to identify the predisposing factors for failure, so that appropriate selection of infants can be done for the future randomized trial.
Methods: A retrospective chart review was done of all the babies who were born in a tertiary hospital between 1st January 2014 to 31st December 2015 (2 years) and received surfactant through INSURE method. Infants requiring reintubation and mechanical ventilation within 3 days post INSURE are considered as INSURE failure for our study purpose.
Results: Eighty-five infants were included in the review with gestational age (GA) ranging from 26+3 to 35+5 weeks and birth weight ranging from 680 to 3340 grams. Of these, 22 infants (26%) had INSURE failure. INSURE failure rate was higher in infants born <30 weeks gestation (40%). Higher FiO2 requirement prior to INSURE (mean FIO2 0.5 vs 0.3, P value <0.001) and preeclampsia in mothers of infants < 30 weeks of GA (P value 0.027) were strongly associated with INSURE failure. No mortality was noted in either group.
Conclusion: We found that INSURE method may be useful in preventing the need for mechanical ventilation in late preterm infants with RDS. However, this method may be less successful in preterm infants with lower GA (<30 weeks) and higher FiO2 requirement (=0.5). More prospective studies are needed to assess the effectiveness of INSURE method.
Keywords: INSURE, respiratory distress syndrome, continuous positive airway pressure, surfactant, chronic lung disease.
Abbreviations
RDS: Respiratory distress syndrome,
CPAP: Continuous positive airway pressure,
CLD: chronic lung disease.
Background
Respiratory distress syndrome (RDS), also known as Hyaline Membrane Disease is a major cause of morbidity and mortality in preterm infants. RDS is a result of surfactant deficiency, which causes increased surface tension in the air-liquid interface of the terminal respiratory units leading to atelectasis, increased ventilation perfusion mismatch, and potential lung injury due to a marked pulmonary inflammatory response [1]. The incidence of RDS increases with decreasing GA, and infants born below 30 weeks GA are at the greatest risk for RDS related complications [2].
Early surfactant treatment reduces mortality, and decreases the incidence of air leaks and chronic lung disease (CLD) in preterm infants at risk of RDS [3-5]. These infants are usually managed with endotracheal intubation and surfactant administration followed by mechanical ventilation however this has immediate and long-term complications. Evidence from animal research showed that mechanical ventilation triggers inflammatory lung injury resulting in CLD, which is decreased with the use of continuous positive airway pressure (CPAP) [6]. Multiple reviews comparing the nasal CPAP and mechanical ventilation have shown that risk of CLD is decreased in CPAP group [7-9]. Other reported complications associated with prolonged intubation and mechanical ventilation include subglottic stenosis [10] or voice abnormalities at school age [11].
Since the introduction of Continuous positive airway pressure (CPAP) support in the treatment of RDS there has been significant reduction in mortality and morbidity.
Gregory et al has shown reduction in mortality from 50% to 20% with the use of CPAP in RDS [12]. Another study showed that the use of CPAP along with the surfactant administration has further reduced the mortality rate from 20% to 10% [13]. Therefore, early use of nasal CPAP and surfactant administration without using mechanical ventilation has been accepted as an alternate method of managing preterm infants with RDS. This can be achieved by INSURE method, which involves endotracheal intubation and surfactant administration followed by extubation to CPAP support, or by less invasive method of surfactant administrations (LISA) through thin endotracheal catheter [14-16]. Non-invasive methods of surfactant administration though pharyngeal instillation [17], laryngeal mask airway [18] and nebulization [19] have also been reported.
INSURE method has been evaluated as the method of initial stabilization in the delivery room or shortly after birth (early INSURE) [20-22] and/or at the later course of RDS when preterm infants had already been commenced on nasal CPAP (late INSURE) [3,23-25]. INSURE method has been compared with various other methods like nasal CPAP without surfactant administration, less invasive surfactant administration (LISA), and conventional standard approach (intubation, surfactant administration and mechanical ventilation) [20,24,26-29]. INSURE method has been shown to reduce the incidence of CLD when compared to conventional approach due to avoidance of mechanical ventilation [30].
INSURE method is not always successful in preterm infants with RDS. Failure rate from INSURE method ranges from 15% - 69% [31-33]. The various risk factors that have been attributed to the failure rate were lower GA, low haemoglobin level, higher oxygen requirement, higher pCO2 level and radiological evidence of severe RDS. We aimed to evaluate the failure rate of INSURE method and to identify the predisposing factors for failure, so that appropriate selection of infants can be done for future randomized trial. We will consider a prospective follow up study based on the results from this retrospective study.
Methods
We conducted a retrospective review of all the preterm babies who were born with RDS and received surfactant through INSURE method in a regional tertiary care level 3 hospital between 1st January 2014 to 31st December 2015 (2 years).
Cases were identified using hospital neonatal database. Relevant clinical details were obtained by reviewing the medical records. Data on antenatal factors that include preeclampsia, antepartum haemorrhage, gestational diabetes, and pregnancy induced hypertension, premature prolonged rupture of membrane (PPROM), antenatal steroids and chorioamnionitis was recorded. Chorioamnionitis is diagnosed clinically in the setting of maternal pyrexia, offensive vaginal discharge and fetal tachycardia [34], and confirmed by histological examination of placenta. Data on delivery details that include mode of delivery, presentation and meconium stained amniotic fluid, and infant’s data that include gestational age, birth weight, gender, multiple pregnancies, APGAR scores and resuscitation required at birth was collected. Further data on infants characteristics prior to INSURE that include respiratory distress status, administration of caffeine, duration of CPAP, highest positive end-expiratory pressure (PEEP), maximum FIO2 required, blood gas analysis results within one hour prior to surfactant administration, radiological diagnosis of RDS, age of infants when the first dose of surfactant was administered and the type of surfactant administered was also collected. Clinical and biochemical data on post INSURE details that were collected include duration of CPAP, highest PEEP, blood gas analysis within 4 hours of surfactant administration, subsequent intubation, mechanical ventilation duration, further dose of surfactant, air leak syndrome, sepsis, necrotizing enterocolitis, patent ductus arteriosus (PDA), retinopathy of prematurity (ROP), intraventricular haemorrhage (IVH), periventricular leukomalacia (PVL), chronic lung disease (CLD) and death during inpatient stay. CLD was defined as the need for respiratory support beyond 36 weeks post-conceptual age [35].
INSURE method has been used regularly in this unit since 2014. All the infants who were treated with INSURE method were identified using the identifiable label in their case record stating this. As per the unit guideline, any preterm infant with respiratory distress needing CPAP support = 7cm H2O and FiO2 = 0.3 to maintain O2 saturation between 91% - 95% and/or blood gas analysis (arterial, venous or capillary) showing respiratory acidosis with pH < 7.25 and pCO2 > 60 mm of Hg, and chest x-ray changes consistent with RDS were eligible for INSURE. Infants who were intubated for apnoea, or received extensive cardiopulmonary resuscitation, and infants who had other medical conditions such as anaemia, congenital heart disease, or chromosomal anomalies were not considered eligible for INSURE method.
Eligible infants were intubated orally as per the standard technique using appropriate size endotracheal tube (ETT), and ETT placement was confirmed by pedicap CO2 detector and auscultation of the chest. Surfactant, either Beractant (survanta) 100 mg/kg or Poractant Alfa (curosurf) 200 mg/kg, was administered in 2 boluses through ETT, and subsequently extubated to nasal CPAP. None of the infants received premedication or induction drugs for intubation as per unit policy. For our study purpose INSURE failure is defined as clinical deterioration along with increasing oxygen requirement with FiO2 = 0.6 and CPAP = 7cm H2O or worsening respiratory acidosis with pH <7.25 and pCO2 >60 mm of Hg or significant apneic episode requiring Bag & Mask ventilation. These infants were reintubated and they received mechanical ventilator support.
Primary outcome was to measure INSURE failure rate in this population and to identify potential causes for INSURE failure. Secondary outcomes measured were air leak syndrome (pulmonary interstitial emphysema, pneumothorax or pneumomediastinum), chronic lung disease, and intraventricular haemorrhage, periventricular leukomalacia, necrotizing enterocolitis and death during inpatient stay.
Approval from the institutional quality improvement (QI) projects committee and research screening committee was obtained prior to study.
Statistical Analysis
For descriptive data analysis, continuous data was presented as median and interquartile range, and categorical data as frequencies and percentages. Comparison of groups were done using Chi square test or Mann-Whitney Rank Sum Test. Sigma Stat v3.5 (SPSS, http://www.spss.com) was used for conducting the above statistics and a P value of <0.05 was considered to be significant.
Results
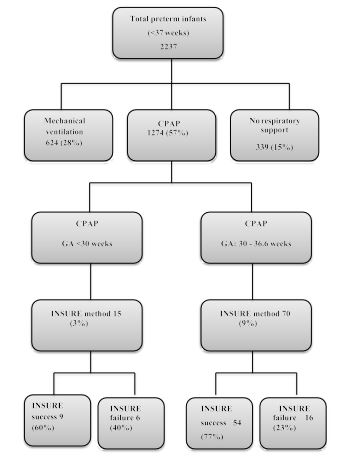
Over the study period, a total of 85 preterm infants received surfactant through INSURE method. Of these, 22 infants (26%) had INSURE failure that required reintubation and received mechanical ventilation. Figure 1 flow chart details the number of preterm infants who received various respiratory support and INSURE during the 2-year review period.
Table 1 illustrates the baseline characteristics of two groups. Most of the variables were comparable between the two groups. GA (median GA 32+4 weeks in both groups) and birth weight (BW) (median BW 1765g vs 1940g) were similar in both groups. Eleven out of 85 infants were noted to have intrauterine growth retardation. There were 52 males, 49 singleton infants and majority were born by cesarean section (69%). All of them had clear liquor at delivery and 81 (out of 85) infants required positive pressure ventilation (PPV) followed by CPAP at birth. Median PEEP (6 cm in both groups) and duration of CPAP support administered (3 hour vs 2 hour) prior to INSURE were similar in both groups. Caffeine was administered in 53 infants (out of 85) and no difference was noted between the groups. There was no difference in carbon dioxide level on blood gas analysis (capillary blood gas) that was performed in 30 infants (out of 85). However, when compared between the groups there was significant difference in FiO2 requirement prior to INSURE. FiO2 requirement was higher in INSURE failure group when compared to INSURE success group (mean FiO2: 0.5 vs 0.3, p value <0.001).
There was no difference for maternal characteristics between groups in our cohort. Sixty (95%) mothers received at least 1 dose of antenatal steroid in INSURE success group vs 18 (81%) in INSURE failure group and the proportion of mothers who received complete course of antenatal steroids was similar in both groups (71% vs 72%). Other maternal risk factors such as pre-eclampsia [16], gestational diabetes [13], antepartum haemorrhage [22], preterm prolonged rupture of membrane [15] and Chorioamnionitis [3] were comparable between groups.
Table 2 summarizes the post INSURE characteristics and comparison of outcomes between the groups. Median age of infants at the time of INSURE administration was similar (3.1 hour vs 2.9 hour) and majority of them received Beractant (89%). In the INSURE failure group, infants were re-intubated at median time of 4 hours post INSURE, all of them received second dose of surfactant and subsequently received mechanical ventilation for mean duration of 22 hours. Blood gas analysis (capillary blood gas) on 50 infants (out of 85) did not show any significant difference in carbon dioxide level. We found a non-significant trend towards longer median duration of CPAP use (122 hours) in INSURE failure group when compared to INSURE success group (59 hours) (P = 0.055). In INSURE failure group there were more number of infants requiring antibiotics and higher incidence of CLD. ROP screening was performed in 25 infants (out of 85) and none of these infants had significant disease warranting treatment. PDA assessment by clinician performed cardiac ultrasound was done in 13 infants (out of 85) and 4 infants showed haemodyanamically significant PDA (all in INSURE failure group). Cranial ultrasound was performed in 55 infants (out of 85) that showed grade 1 unilateral IVH (Volpe’s classification) in four infants and PVL in 1 infant. Seven infants were found to have air leak syndrome (pneumothorax in 4, pneumothorax and pulmonary interstitial emphysema in 2, and pneumomediastinum in 1). There was no episode of necrotizing enterocolitis, or neonatal death in either group.
Subgroup analysis was performed for infants with GA < 30 weeks. Our cohort included 15 infants with GA < 30 weeks, of these 14 were born at GA between 28 weeks and 30 weeks and one infant was born at 26.6 weeks gestation. Nine out of 15 were in the INSURE success group (60%). All the previously mentioned risk factors were assessed for this subgroup of infants, which showed pre-eclampsia as an association for INSURE failure (p= 0.027)
Figure 1:Flow diagram detailing the respiratory supports received by preterm infants (1st Jan 2014-31st> Dec 2015).
| Variable | INSURE success n (%) (n=63) | INSURE failure n (%) (n=22) | p-value |
|---|---|---|---|
| Gestation: 30 – 36.6 weeks | 54 (77%) | 16 (23%) | N/A |
| <30 weeks | 9 (60%) | 6 (40%) | N/A |
| Birth weight (grams) | 1765 (1443–2148) | 1940 (1420–2355) | 0.41 |
| IUGR | 8 (12%) | 2 (9%) | 0.87 |
| Females | 27 (42%) | 6 (27%) | 0.18 |
| Maternal comorbidities | |||
| Preeclampsia | 11 (17%) | 5 (22%) | 0.61 |
| Gestational diabetes | 10 (15%) | 3 (13%) | 0.8 |
| APH | 17 (26%) | 5 (22%) | 0.69 |
| PPROM | 10 (15%) | 5 (22%) | 0.5 |
| Chorioamnionitis | 2 (3%) | 1 (4%) | 0.78 |
| Antenatal steroids: any dose | 60 (95%) | 18 (81%) | 0.14 |
| Complete | 45 (71%) | 16 (72%) | 0.78 |
| Multiple pregnancy | |||
| Singleton | 35 (55%) | 14 (63%) | 0.51 |
| Multi | 28 (44%) | 8 (36%) | 0.51 |
| Mode of delivery | |||
| Vaginal delivery | 21 (33%) | 5 (22%) | 0.16 |
| Cesarean section | 42 (66%) | 17 (77%) | 0.33 |
| Apgar | |||
| at 1 minute | 7 (6 – 8) | 7.5 (6 – 9) | 0.71 |
| at 5 minutes | 9 (8 – 9) | 9 (8 – 9) | 0.74 |
| Delivery room resuscitation | |||
| CPAP or PPV | 62 (99%)22 (100%) | 22 (100%) | 0.32 |
| Not required | 1 (1%) | 0 | 0.32 |
| Categorical data: number (%); Continuous data: Median (IQR) CPAP: Continuous positive airway pressure, PPV: Positive pressure ventilation, IUGR: Intrauterine growth retardation, APH: Antepartum hemorrhage, PPROM: Preterm prolonged rupture of membrane, N/A: not applicable, NS: Not significant | |||
Table 1: Pre-INSURE characteristics: comparison and its significance.
| Variable | INSURE success n (%) (n=63) | INSURE failure n (%) (n=22) | p-value |
|---|---|---|---|
| Age at INSURE (hours) | 3.1 (2.3 – 5.5) | 2.9 (1.9 – 4.1) | 0.69 |
| Surfactant type | |||
| Beractant (survanta) | 56 (88%) | 20 (90%) | 0.78 |
| Poractant alfa (curosurf) | 7 (11%) | 2 (9%) | 0.78 |
| Blood gas (total – 50) | |||
| pH | 7.28 (7.23 – 7.34) | 7.26 (7.2 – 7.3) | 0.44 |
| pCO2(mmHg) | 53 (43 – 61) | 57 (43 – 68) | 0.56 |
| BE (mmol/L) | -2 ((-3) – (-1)) | -3 ((-4) – (-1)) | 0.43 |
| CPAP duration (hours) | 59 (25 – 158) | 122 (41 – 495) | 0.055 |
| Time at reintubation (hours) | N/A | 4 (1 – 16) | N/A |
| Surfactant 2nd dose given | N/A | 22 (100%) | N/A |
| MV duration (hours) | N/A | 22 (9 – 69) | N/A |
| Antibiotics ≥ 5 days | 7 (11%) | 10 (45%) | <0.001 |
| Air leak syndrome | 4 (6%) | 3 (13%) | 0.37 |
| Necrotizing enterocolitis | 0 | 0 | N/A |
| Patent ductus arteriosus | 2 (3%) – out of 4 | 8 (36%) – out of 9 | N/A |
| Intraventricular hemorrhage | 2 (3%) – out of 42 | 2 (9%) – out of 13 | N/A |
| Periventricular leukomalacia | 0 – out of 42 | 1 (4%) – out of 13 | N/A |
| Chronic lung disease | 0 | 5 | <0.001 |
| Retinopathy of prematurity | 0 – out of 15 | 0 – out of 10 | N/A |
| Neonatal death | 0 | 0 | N/A |
| Categorical data: number (%); Continuous data: Median (IQR) CPAP: Continuous positive airway pressure, MV: Mechanical ventilation, N/A: not applicable, NS:Not significant | |||
Table 2: Post INSURE characteristics and comparison of outcomes.
Discussion
Our retrospective study of 85 preterm infants (<37 weeks of GA) who received surfactant through INSURE method showed INSURE failure rate of 26 % and this rose to 40% if we consider infants with GA <30 weeks only. We found that higher FiO2 requirement (prior to INSURE) and pre-eclampsia (in mothers of infant with GA<30 weeks) were strongly associated with failure of INSURE (P values <0.001 and 0.027 respectively).
INSURE failure rate in other studies range from 15% - 69% [31,32,36-38]. The wide range is probably secondary to variation in the included study population and variable threshold point for reintubation. Brix et al reported the highest incidence of INSURE failure rate (69%). In their cohort, 51%, (162 out of 322) were extreme premature infants (<28 weeks gestation). They have noted failure rate of 54% for infants with GA 28 – 32 weeks and 84% for infants with GA <28 weeks. Similarly, we also noted increase in failure rate for preterm infants with lower gestational age (22% for infants with GA = 30 weeks gestation vs 40% for infants with GA <30 weeks).
The study by Cherif et al [32] is one of the first studies to identify the risk factors for Insure failure, which included infants from 27 weeks to 34 weeks of GA (total 109), and had failure rate of 32%. They reported that the arterial partial pressure of carbon dioxide (mean pCO2 55.4 vs 41.4, adjusted odds ratio [OR] = 1.82; 95% confidence interval [CI] = 1.76 to 90.56), mean arterial-to-alveolar oxygen tension ratio (mean a/ApO2 0.16 vs 0,24, adjusted OR = 1.13; 95% CI = 1.06 to 85.34) and severe radiological grade (adjusted OR = 1.31; 95% CI = 1.15 to 70.16) as independent predictors for INSURE failure. Subsequent studies have also showed higher oxygen requirement is associated with INSURE failure [31,36,37]. Cochrane review by Stevens et al concluded that treatment with surfactant by transient intubation using a low treatment threshold (FiO2< 0.45) is preferable [4]. Similar to the above studies, we noted higher FiO2 is associated with INSURE failure.
Randomized trial on INSURE method vs CPAP support without surfactant administration showed that there was no benefit in mortality and morbidity when the surfactant was administered at the infant’s mean age of 5.1 hours in INSURE group [39]. In our cohort, the surfactant was administered at infant’s mean age of 4.7 hours, which was similar in both groups (mean age of 4.8 hour vs 4.3 hour).
Cochrane review by Subramaniam et al, comparing CPAP support and assisted ventilation, found that, in CPAP group, there was a small but clinically significant reduction in the incidence of CLD at 36 weeks (typical RR 0.89, 95% CI 0.79 to 0.99; typical RD -0.04, 95% CI -0.08 to 0.00; 3 studies, 772 infants, moderate-quality evidence); and decreased death or BPD (typical RR 0.89, 95% CI 0.81 to 0.97; typical RD -0.05, 95% CI -0.09 to 0.01; 3 studies, 1042 infants, moderate-quality evidence) [8]. In our cohort there were 5 infants with CLD in the INSURE failure group compared to none in the INSURE success group (P value <0.001).
We also noted pre-eclampsia as an association for INSURE failure in infants below 30 weeks of GA (p=0.027), which is contrary to the finding noted by Brix et al [31], who reported that pre-eclampsia decreased the risk of INSURE failure. Possible explanation is that pre-eclampsia compromises uterine blood flow resulting in hypoxia and ischaemia which may restrict fetal angiogenesis and suppress the development of alveolarization (“vascular hypothesis of BPD”) [40]. Further study in maternal pre- eclampsia and fetal lung-vascular pathophysiology might shed light on pulmonary vascular development in infants with RDS.
The main aim of INSURE method is to prevent mechanical ventilation and avoid lung injury, and thus decreasing the morbidity and mortality. This has been indicated by multiple RCT’s [20-22,24-26,41,42] and our study also indicates this benefit as there were decreased incidence of CLD in infants who were successful on INSURE.
Even though our study is retrospective in nature, it has assessed large number of well- matched maternal and infant characteristics. Our study did not have comparator and had small number of infants with GA < 30 weeks. We did not repeat the INSURE for the infants who were unsuccessful on the first attempt of INSURE.
Conclusion
One fourth of our preterm population who received surfactant through INSURE method required reintubation and mechanical ventilation. Higher FIO2 requirement prior to INSURE and maternal pre-eclampsia were strongly associated with INSURE failure.
We conclude that INSURE method may be useful in avoiding mechanical ventilation in late preterm infants with RDS, however this method may be less successful in infants with lower gestational age (<30 weeks) and higher FiO2 requirement (=0.5). More prospective studies are required to further assess the effectiveness of INSURE method.
Acknowledgement
None.
Funding
None.
Availability of Data and Materials
All the data of this study are available from the corresponding author upon reasonable request.
Authors’ Contributions
SA and DW conceptualised the study. MS and SA analysed and interpreted the patient data. MS was a major contributor in writing the manuscript. All authors (MS, SA, DW) revised the manuscript for important intellectual content and gave final approval of the version to be published.
Disclosure of Previous Presentation
Portions of this study were presented as an e-poster discussion at the Perinatal Society of Australia and New Zealand (PSANZ) Annual Congress 2017
Ethical declarations
Ethics approval and consent to participate
The study protocol was approved by the institutional review board of King Edward Memorial Hospital. The need for informed consent was waived on account of the retrospective nature of the demographic, clinical, and outcome data. All patients’ data were de-identified prior to the analysis.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
Clark RH, Gerstmann DR, Jobe AH, Moffitt ST, Slutsky AS, et al. (2001) Lung injury in neonates: causes, strategies for prevention, and long-term consequences. The Journal of pediatrics 139: 478-486.[ Ref ]
Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, et al. (2010) Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 126: 443-456.[ Ref ]
Bahadue FL, Soll R (2012) Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. The Cochrane database of systematic reviews 11: Cd001456.[ Ref ]
Stevens TP, Harrington EW, Blennow M, Soll RF (2007) Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. The Cochrane database of systematic reviews.[ Ref ]
Yost CC, Soll RF (2000) Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. The Cochrane database of systematic reviews.[ Ref ]
Jobe AH, Kramer BW, Moss TJ, Newnham JP, Ikegami M (2002) Decreased indicators of lung injury with continuous positive expiratory pressure in preterm lambs. Pediatric research 52: 387-392.[ Ref ]
Committee on Fetus and Newborn, American Academy of Pediatrics (2014) Respiratory support in preterm infants at birth. Pediatrics 133: 171-174.[ Ref ]
Subramaniam P, Ho JJ, Davis PG (2016) Prophylactic nasal continuous positive airway pressure for preventing morbidity and mortality in very preterm infants. The Cochrane database of systematic reviews. 14: Cd001243.[ Ref ]
Schmolzer GM, Kumar M, Pichler G, Aziz K, O’Reilly M, et al. (2013) Noninvasive versus invasive respiratory support in preterm infants at birth: systematic review and meta-analysis. BMJ (Clinical research ed) 347: f5980.[ Ref ]
Johnson LB, Rutter MJ, Shott SR, Cotton RT (2005) Acquired subglottic cysts in preterm infants. The Journal of otolaryngology 34: 75-78.[ Ref ]
French N, Kelly R, Vijayasekaran S, Reynolds V, Lipscombe J, et al. (2013) Voice abnormalities at school age in children born extremely preterm. Pediatrics 131: e733-e739.[ Ref ]
Gregory GA, Kitterman JA, Phibbs RH, Tooley WH, Hamilton WK (1971) Treatment of the idiopathic respiratory-distress syndrome with continuous positive airway pressure. The New England journal of medicine 284: 1333-1340.[ Ref ]
Morley C, Gore S, Raju TK, Vidyasagar D, Levy PS (1987) Surfactants in severe hyaline membrane disease. Lancet (London, England) 1: 1040- 1041.[ Ref ]
Gopel W, Kribs A, Hartel C, Avenarius S, Teig N, et al. (2015) Less invasive surfactant administration is associated with improved pulmonary outcomes in spontaneously breathing preterm infants. Acta paediatrica 104: 241-246[ Ref ]
Kribs A, Roll C, Gopel W, Wieg C, Groneck P, et al. (2015) Nonintubated Surfactant Application vs Conventional Therapy in Extremely Preterm Infants: A Randomized Clinical Trial. JAMA pediatrics 169: 723-730.[ Ref ]
Gopel W, Kribs A, Ziegler A, Laux R, Hoehn T, et al. (2011) Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, randomised, controlled trial. Lancet 378: 1627-1634.[ Ref ]
Kattwinkel J, Robinson M, Bloom BT, Delmore P, Ferguson JE (2004) Technique for intrapartum administration of surfactant without requirement for an endotracheal tube. Journal of perinatology: official journal of the California Perinatal Association 24: 360-365.[ Ref ]
Pinheiro JM, Santana-Rivas Q, Pezzano C (2016) Randomized trial of laryngeal mask airway versus endotracheal intubation for surfactant delivery. Journal of perinatology: official journal of the California Perinatal Association 36: 196-201.[ Ref ]
Finer NN, Merritt TA, Bernstein G, Job L, Mazela J et al. (2010) An open label, pilot study of Aerosurf(R) combined with nCPAP to prevent RDS in preterm neonates. Journal of aerosol medicine and pulmonary drug delivery 23: 303-309.[ Ref ]
Dunn MS, Kaempf J, de Klerk A, de Klerk R, Reilly M, et al. (2011) Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics 128: e1069-1076.[ Ref ]
Rojas MA, Lozano JM, Rojas MX, Laughon M, Bose CL, et al. (2009) Very early surfactant without mandatory ventilation in premature infants treated with early continuous positive airway pressure: a randomized, controlled trial. Pediatrics 123: 137-142.[ Ref ]
Sandri F, Plavka R, Ancora G, Simeoni U, Stranak Z, et al. (2010) Prophylactic or early selective surfactant combined with nCPAP in very preterm infants. Pediatrics 125: e1402-1409.[ Ref ]
Okulu E, Arsan S, Mungan Akin I, Alan S, Kilic A, et al. (2015) Early or later prophylactic INSURE in preterm infants of less than 30 weeks’ gestation. The Turkish journal of pediatrics 57: 1-8.[ Ref ]
Dani C, Bertini G, Pezzati M, Cecchi A, Caviglioli C, et al. (2004) Early extubation and nasal continuous positive airway pressure after surfactant treatment for respiratory distress syndrome among preterm infants <30 weeks’ gestation. Pediatrics 113: e560-563.[ Ref ]
Reininger A, Khalak R, Kendig JW, Ryan RM, Stevens TP, et al. (2005) Surfactant administration by transient intubation in infants 29 to 35 weeks’ gestation with respiratory distress syndrome decreases the likelihood of later mechanical ventilation: a randomized controlled trial. Journal of perinatology: official journal of the California Perinatal Association 25: 703-708.[ Ref ]
Nayeri FS, Esmaeilnia Shirvani T, Aminnezhad M, Amini E, Dalili H, et al. (2014) Comparison of INSURE method with conventional mechanical ventilation after surfactant administration in preterm infants with respiratory distress syndrome: therapeutic challenge. Acta medica Iranica 52: 596-600.[ Ref ]
Isayama T, Iwami H, McDonald S, Beyene J (2016) Association of Noninvasive Ventilation Strategies With Mortality and Bronchopulmonary Dysplasia Among Preterm Infants: A Systematic Review and Meta-analysis. JAMA 316: 611- 624.[ Ref ]
Ali E, Abdel Wahed M, Alsalami Z, Abouseif H, Gottschalk T, et al. (2016) New modalities to deliver surfactant in premature infants: a systematic review and meta-analysis. The journal of maternal-fetal & neonatal medicine 29: 3519-324.[ Ref ]
Kanmaz HG, Erdeve O, Canpolat FE, Mutlu B, Dilmen U (2013) Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial. Pediatrics 131: e502-509.[ Ref ]
Pfister RH, Soll RF (2012) Initial respiratory support of preterm infants: the role of CPAP, the INSURE method, and noninvasive ventilation. Clinics in perinatology 39: 459-481.[ Ref ]
Brix N, Sellmer A, Jensen MS, Pedersen LV, Henriksen TB (2014) Predictors for an unsuccessful INtubation-SURfactant-Extubation procedure: a cohort study. BMC pediatrics 14: 155.[ Ref ]
Cherif A, Hachani C, Khrouf N (2008) Risk factors of the failure of surfactant treatment by transient intubation during nasal continuous positive airway pressure in preterm infants. American journal of perinatology 25: 647-652.[ Ref ]
Dani C, Berti E, Barp J (2010) Risk factors for INSURE failure in preterm infants. Minerva pediatrica 62(S1): 19-20.[ Ref ]
Royal College of Obstetricians and Gynecologists (2006) Preterm Prelabour Rupture of Membranes. Guideline No. 44.[ Ref ]
Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM (1988) Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 82: 527-532.[ Ref ]
Dani C, Corsini I, Poggi C (2012) Risk factors for intubation-surfactantextubation (INSURE) failure and multiple INSURE strategy in preterm infants. Early human development 88 S1:S3-4.[ Ref ]
Li T, Jiang H, Liu DY, Li XH (2014) Risk factors for the failure of the InSure method in very preterm infants with respiratory distress syndrome. Chinese journal of contemporary pediatrics 16: 610-613.[ Ref ]
Naseh A, Yekta BG (2014) INSURE method (INtubation-SURfactantExtubation) in early and late premature neonates with respiratory distress: factors affecting the outcome and survival rate. The Turkish journal of pediatrics 56: 232-237.[ Ref ]
Nakhshab M, Tajbakhsh M, Khani S, Farhadi R (2015) Comparison of the effect of surfactant administration during nasal continuous positive airway pressure with that of nasal continuous positive airway pressure alone on complications of respiratory distress syndrome: a randomized controlled study. Pediatrics and neonatology 56: 88-94.[ Ref ]
Backes CH, Markham K, Moorehead P, Cordero L, Nankervis CA, et al. (2011) Maternal Preeclampsia and Neonatal Outcomes. Journal of Pregnancy 2011: 214365.[ Ref ]
Tooley J, Dyke M (2003) Randomized study of nasal continuous positive airway pressure in the preterm infant with respiratory distress syndrome. Acta Paediatrica 92: 1170-1174.[ Ref ]
Verder H, Albertsen P, Ebbesen F, Greisen G, Robertson B, et al. (1999) Nasal continuous positive airway pressure and early surfactant therapy for respiratory distress syndrome in newborns of less than 30 weeks’ gestation. Pediatrics 103: E24.[ Ref ]