Journal Name: Journal of Pediatrics and Infants
Article Type:Research
Received date:02 February, 2021
Accepted date:30 June, 2021
Published date:01 July, 2021
Citation:Jerzynska J, Smejda K, Borkowska A1 (202) Interleukin-33 and Epitopes in Children Allergic to Cats. J Pediat Infants Vol: 4, Issu: 2 (01-07).
Copyright:© 2020 Jerzynska J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: The prevalence of allergy to furry animals has been increasing. An important factor in induction of allergic inflammation is interleukin-33 (Il-33). Molecular based diagnosis of allergies is an important advancement in improving sensitivity and specificity.
Objective: The study included 51 children aged 5-18 years, allergic to cats, under the care of an allergy clinic.
Method:The current child health status assessment was performed by an allergist. Children were evaluated for the presence of bronchial asthma, atopic dermatitis, allergic rhinitis. A questionnaire evaluating the occurrence of allergic symptoms after contact with the cat and dog was performed. All children were also assessed for the occurrence of the above-mentioned allergic symptoms after contact with the dog. Mothers also completed a questionnaire regarding cat and dog exposure: a) during pregnancy, b) later in life (in the first two years of the child’s life and over the age of 2 years).
From all children, a blood sample was taken to measure IgE serum levels to cat and dog epitopes and the level of Il-33 in the serum. IgE serum levels were determined to the following cat epitopes: Fel d (1,2,4), and dog components: Can f (1,2,3,5).
Results:We have shown that when cat and dog epitopes were present at the same time, there was a correlation with the level of Il-33: negative with cat epitopes (p = 0.027) and positive with dog epitopes (p<0.001). The presence of Fel d 2 and Can f 3 was associated with a lower level of Il-33, whereas the presence of Can f 5 significantly increased the level of Il-33. We demonstrated a positive correlation between the level of Il-33, and the number of dog epitopes (p = 0.004) but not with the number of cat epitopes (p = 0.814).
Conclusion: The identification of biomarkers of allergic diseases may be important for the diagnosis and treatment of the future.
Keywords: Children, Il-33, Cat sensitization, Epitopes.
Abstract
Background: The prevalence of allergy to furry animals has been increasing. An important factor in induction of allergic inflammation is interleukin-33 (Il-33). Molecular based diagnosis of allergies is an important advancement in improving sensitivity and specificity.
Objective: The study included 51 children aged 5-18 years, allergic to cats, under the care of an allergy clinic.
Method:The current child health status assessment was performed by an allergist. Children were evaluated for the presence of bronchial asthma, atopic dermatitis, allergic rhinitis. A questionnaire evaluating the occurrence of allergic symptoms after contact with the cat and dog was performed. All children were also assessed for the occurrence of the above-mentioned allergic symptoms after contact with the dog. Mothers also completed a questionnaire regarding cat and dog exposure: a) during pregnancy, b) later in life (in the first two years of the child’s life and over the age of 2 years).
From all children, a blood sample was taken to measure IgE serum levels to cat and dog epitopes and the level of Il-33 in the serum. IgE serum levels were determined to the following cat epitopes: Fel d (1,2,4), and dog components: Can f (1,2,3,5).
Results:We have shown that when cat and dog epitopes were present at the same time, there was a correlation with the level of Il-33: negative with cat epitopes (p = 0.027) and positive with dog epitopes (p<0.001). The presence of Fel d 2 and Can f 3 was associated with a lower level of Il-33, whereas the presence of Can f 5 significantly increased the level of Il-33. We demonstrated a positive correlation between the level of Il-33, and the number of dog epitopes (p = 0.004) but not with the number of cat epitopes (p = 0.814).
Conclusion: The identification of biomarkers of allergic diseases may be important for the diagnosis and treatment of the future.
Keywords: Children, Il-33, Cat sensitization, Epitopes.
Introduction
Dog and cat allergens are a common cause of allergic sensitization and triggering respiratory symptoms worldwide. In the ECAP study it was observed that in the Polish population allergy to cat allergens is on average 13.5%, and for a dog 9.7% [1]. According to the latest research, over half of Poles (52%) have any pet in their household. Most often it is a dog or a cat. Forty two percent of Poles declare that they have a dog, 26% cat, and 5% other animals [2].
Molecular based diagnosis of allergies is an important advancement in improving sensitivity and specificity [3]. The major cat allergen is Fel d 1, a uteroglobin [4]. It is produced in the skin and salivary glands [5,6] and is the sensitizing allergen in up to 95% [7]. Other allergens are serum albumin Fel d 2 [8], which is important for crossreactivity to serum albumins of other animals, and lipocalin Fel d 4 [9], which cross-reacts with lipocalin allergens from other animals [10]. Further cat allergens have also been characterized, such as cystatin Fel d 3 [11], cat IgA Fel d 5 and IgM Fel d 6 [12], lipocalin Fel d 7 and latherin-like Fel d 8 [13]. As dog allergens have been identified, seven proteins were designated serially as Can f 1 to Can f 7. Can f 1, Can f 2, Can f 4, and Can f 6 are members of the lipocalin family, which is composed of various secretory lipid-transport proteins [14]. On the other hand, Can f 3, Can f 5, and Can f 7 have been classified as serum albumin, urinary kallikrein, and epididymal secretory protein [15,16]. In allergology, lipocalins represent a significant protein group as major mammalian respiratory allergens from dogs, cats and mice belong to this family [17,18]. Of the lipocalin allergens, Can f 1 was reported as a major dog allergen, where no less than 50–75% of dog-allergic subjects were sensitized [18].
The prevalence of allergy to furry animals has been increasing. An important factor in induction of allergic inflammation is interleukin-33 (Il-33), a member of the interleukin-1 (Il-1) cytokine family [19]. Il-33 is a tissuederived nuclear cytokine produced by endothelial cells, epithelial cells, fibroblast-like cells, and myofibroblasts. Il-33 needs the specific receptor ST2 (membrane-bound receptor) and Interleukin-1 receptor accessory protein heterodimer for its binding, which instigates the production of different types of cytokines and chemokines that have crucial roles in the exacerbation of allergic diseases and inflammation [20,21]. Il-33 acts not only as a Th2-inducing cytokine, but also as a proinflammatory cytokine in various other immune responses [22-24].
The present study aimed to determine the relationship between interleukin-33 and IgE cat and dog components as well as clinical symptoms in children sensitive to cat allergen.
Patients and Methods
Patients
The study included 51 children aged 5-18 years, Caucasian, allergic to cat, under the care of our allergy clinic. They were considered to have a cat allergy if they suffered from one or more of allergy symptoms during contact with the cat; sensitization to cat allergen was confirmed by skin prick tests or specific IgE. All patients were also tested for allergy to house dust mites, mold, pollen, and other animals by skin prick tests or specific IgE. The current child health status assessment was performed by an allergist at the study visit. Children were evaluated for the presence of bronchial asthma, atopic dermatitis, and allergic rhinitis. The definition of asthma used was based on the Global Initiative for Asthma (GINA) guidelines [25]. Atopic dermatitis was diagnosed according to the revised Hanifin and Rajka criteria [26]. The diagnosis of allergic rhinitis was performed according to ARIA guidelines [27].
Study design
There was one study visit. A questionnaire evaluating the occurrence of allergic symptoms, such as: eye symptoms (tearing, redness, pruritus), nasal symptoms (sneezing, nasal itch, runny nose), skin changes, symptoms from the side lower respiratory tract (cough, shortness of breath, wheezing) in children after contact with the cat and dog was performed. Mothers completed also a questionnaire regarding cat exposure: a) during pregnancy (permanently at home or periodically in other people who have a cat), b) having a cat at home in the first two years of the child’s life and over the age of 2 years. All children were also assessed for the occurrence of the above-mentioned allergic symptoms after contact with the dog. Mothers completed analogous questionnaires regarding contact with the dog during pregnancy and after childbirth. Information about having other animals at home was also collected and species of pet possessed (rabbit, hamster, guinea pig, fish, birds). At the study visit, from all children, a blood sample was taken to measure the IgE serum levels to cat and dog epitopes and the level of Il-33 in the serum. In all children IgE serum levels were determined to the following cat epitopes: Fel d 1, Fel d 2, Fel d 4, and dog components: Can f 1, Can f 2, Can f 3, Can f 5.
Laboratory measurements
Commercial enzyme-linked immunosorbent assays (ELISA) were applied to measure serum levels of Il-33 (Biorbyt, USA). The assay was conducted using the protocols recommended by the manufacturers (standard range: 15.6– 1000 pg/ml sensitivity: 4.14 pg/ml).
IgE serum levels to cat and dog components above 0.35 IU/ml were defined as a positive reaction. Serum samples were analysed for IgE antibodies using the ImmunoCAP® system according to the manufacturer’s guidelines (Phadia AB, Uppsala, Sweden). Serum samples from venous blood were used. The samples were stored at -20 °C. ImmunoCAP Specific IgE detects IgE antibodies in the range 0 to 100 kUA /l, where A represents allergen-specific antibodies. The result is reported quantitatively. In clinical practice, 0.35 kUA /l has commonly been used as a cut-off. Clinical performance is expressed as sensitivity, ranging from 84-95 %, and specificity, ranging from 85-94%.
The study was approved by the Ethical Committee of the Medical University, Lodz, Poland; RNN/39/14/KE; written consent was obtained from all the subjects before the study.
Statistical analysis
The numerical variables were described by way of measures of location – arithmetic or geometric mean; measures of dispersion – standard deviation, 95% confidence interval, and minimum and maximum values. The categorical variables were depicted through absolute numbers and percentages. Multivariate logistic regression models (for binary dependent variables such as e.g. allergy symptoms) were carried out in order to test statistical relationships. Generalized linear models with robust standard errors (i.e. sandwich estimators) were fitted for numerical dependent variables (e.g. Il-33 levels). Il-33 concentrations had been natural log transformed prior to run the analyses. Some variables, which revealed the perfect prediction (i.e., when each study participant or none of them displayed a specific trait), were excluded from the regression equations. All the models were controlled for the studied patients’ age and gender. A level of P < 0.05 was deemed statistically significant. All the statistical computations were carried out by means of Stata/Special Edition, release 14.2 (StataCorp LP, College Station, Texas, USA).
Results
Baseline characteristic of the studied patients is presented in Table 1.
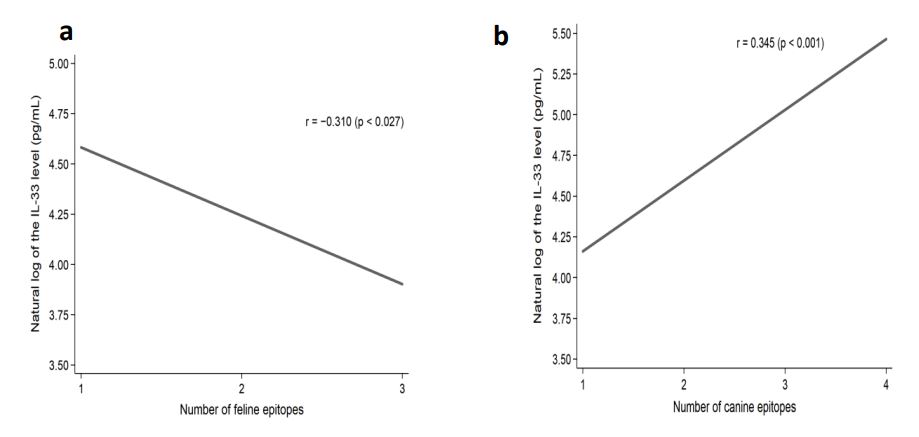
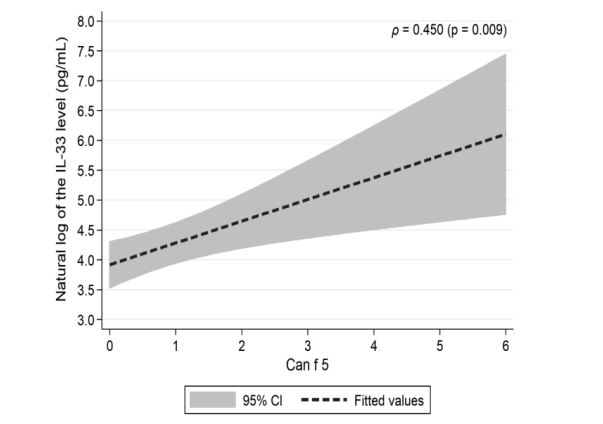
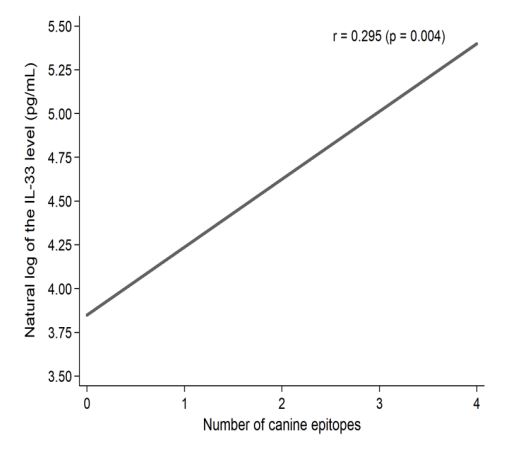
We have shown that when cat and dog epitopes were present at the same time, there was a correlation with the level of Il-33: negative with cat epitopes (p = 0.027) (Figure 1a), and positive with dog epitopes (p <0.001) (Figure 1b). The level of Il-33 significantly increased in children who had any dog epitope and contact with a dog, regardless of symptoms occurrence (p = 0.002). However, in patients with symptoms from the skin after contact with a dog - concentration of IL-33 was significantly higher (p <0.023) (Table 2). The presence of Fel d 2 and Can f 3 was associated with a lower level of Il-33, whereas the presence of Can f 5 significantly increased the level of Il-33 (Table 3). A positive correlation between the IgE level for the Can f 5 epitope and the concentration of IL-33 was demonstrated (p = 0.009) (Figure 2). We demonstrated a positive correlation between the level of Il-33, and the number of dog epitopes (p = 0.004) (Figure 3) but not with the number of cat epitopes (p = 0.814).
There was no statistical relationship between the level of Il-33 and the occurrence of any symptoms after contact with the cat, both in children who had and did not have epitopes for the cat (4 from 51 children). There was no relationship between Il-33 concentration and any contact with cat or dog at home (either permanent or occasional) (Figure 3). We didn’t show a correlation between the level of Il-33, the number of epitopes for cat and dog, and the occurrence of asthma (p = 0.651) or atopic dermatitis (p = 0.274).
| Investigated trait | Statistical parameter | |||
|---|---|---|---|---|
| M | SD | 95% CI | Min. – max. | |
| Age (years) | 11,9 | 3,40 | 10,9-12,8 | 6-18 |
| IL-33 (pg/ml) | 137,7 | 195,5 | 82,8-192,7 | 6,7-952,6 |
| Gender (F/M) | N | % | N | % |
| 18 | 35,3 | 33 | 64,7 | |
| Symptoms after contact with the cat (Y/N) | 39 | 76,5 | 12 | 23,5 |
| Symptoms after contact with the dog (Y/N) | 26 | 51,0 | 25 | 49,0 |
| Presence of other pets (Y/N) | 7 | 13,7 | 44 | 86,3 |
| Asthma (Y/N) | 46 | 90,2 | 5 | 9,8 |
| Allergic rhinitis (Y/N) | 51 | 100,0 | 0 | 0,0 |
| Atopic dermatitis (Y/N) | 6 | 11,8 | 45 | 88,2 |
Table 1: Baseline characteristics of the studied patients (n = 51).
| Symptoms after contact with dog/cat | Symptoms present | Symptoms absent | Level of statistical significance (p-value)** | ||||||
|---|---|---|---|---|---|---|---|---|---|
| M* | SD | 95% CI | Min.-max | M* | SD | 95% CI | Min.-max | ||
| Cat – any | 64,20 | 1,33 | 41,44-99,46 | 6,70-952,60 | 69,52 | 0,97 | 37,50-128,90 | 21,70-338,60 | = 0,292 |
| Cat – ocular | 63,09 | 1,18 | 40,86-97,44 | 8,20-952,60 | 69,45 | 1,37 | 35,86-134,51 | 6,70-789,60 | = 0,645 |
| Cat – nasal | 66,73 | 1,28 | 42,01-106,01 | 6,70-952,60 | 63,20 | 1,21 | 34,60-115,43 | 8,20-469,70 | = 0,563 |
| Cat – cutaneous | 75,64 | 1,04 | 35,89-159,41 | 18,10-469,70 | 66,50 | 1,27 | 43,99-100,53 | 6,70-952,60 | = 0,730 |
| Cat – lower airways | 58,63 | 1,29 | 25,75-133,49 | 11,30-952,60 | 67,75 | 1,25 | 44,98-102,04 | 6,70-789,60 | = 0,729 |
| Dog – any | 73,97 | 1,41 | 30,27-180,76 | 11,40-952,60 | 62,96 | 1,21 | 42,31-93,67 | 6,70-789,60 | = 0,497 |
| Dog – ocular | 79,24 | 1,76 | N/A | 16,10-952,60 | 64,36 | 1,22 | 44,84-92,36 | 6,70-789,60 | = 0,336 |
| Dog – nasal | 78,71 | 1,50 | 19,60-316,15 | 11,40-952,60 | 63,50 | 1,22 | 43,66-92,36 | 6,70-789,60 | = 0,454 |
| Dog – cutaneous | 131,72 | 0,74 | N/A | 63,80-370,5 | 60,54 | 1,27 | 41,32-88,72 | 6,70-952,60 | = 0,023 |
| Dog – lower airways | 91,67 | 1,39 | 25,35-331,58 | 12,10-952,60 | 61,94 | 1,23 | 42,42-90,45 | 6,70-789,60 | = 0,385 |
| (*Geometric means were provided. ** Main effects were tested.) | |||||||||
Table 2: Blood concentrations of Il-33 (pg/mL) in the studied patients by presence of symptoms after contact with the cat and/or with the dog (n = 50) (presented as mean, standard deviation, 95% confidence interval, and min.-to-max. values).
| Epitope | Epitope present | Epitope absent | Level of statistical significance (p-value)** | ||||||
|---|---|---|---|---|---|---|---|---|---|
| M* | SD | 95% CI | Min.-max | M* | SD | 95% CI | Min.-max | ||
| Fel d 1 | 65,25 | 1,27 | 44,57-95,51 | 6,70-952,60 | 67,19 | 1,15 | N/A | 11,30-206,60 | = 0,342 |
| Fel d 2 | 44,22 | 1,17 | N/A | 6,70-153,90 | 68,35 | 1,26 | 46,83-99,77 | 8,20-952,60 | = 0,041 |
| Fel d 4 | 55,44 | 1,34 | 23,71-129,60 | 6,70-552,40 | 68,96 | 1,23 | 46,02-103,32 | 8,20-952,60 | = 0,682 |
| Can f 1 | 97,51 | 1,37 | 46,88-202,81 | 6,70-789,60 | 54,24 | 1,15 | 36,25-81,15 | 11,40-952,60 | = 0,138 |
| Can f 2 | 93,59 | 1,42 | 21,00-417,13 | 12,10-552,40 | 62,32 | 1,23 | 42,88-90,59 | 6,70-952,60 | = 0,516 |
| Can f 3 | 37,21 | 1,13 | 11,38-121,73 | 6,70-153,90 | 70,67 | 1,25 | 48,28-103,45 | 8,20-952,60 | = 0,012 |
| Can f 5 | 144,97 | 1,20 | 74,61-281,69 | 6,70-552,40 | 47,38 | 1,13 | 31,97-70,23 | 8,20-952,60 | = 0,030 |
| (*Geometric means were provided. ** Main effects were tested.) | |||||||||
Table 3: Blood concentrations of Il-33 (pg/mL) in the studied patients by presence of selected epitopes from the Fel d and Can f family (n = 50) (presented as mean, standard deviation, 95% confidence interval, and min.-to-max. values).
Figure 1: Correlation of natural log of the IL-33 level (pg/mL) and the number of IgE epitopes for cat (a) and dog (b) epitopes present at the same time in the studied patients.
Figure 2: Correlation of natural log of the IL-33 level (pg/mL) and Can f 5 epitope level in the studied patients.
Figure 3: Correlation of natural log of the IL-33 level (pg/mL) and the number of IgE epitopes for the dog in the studied patients.
Discussion
Il-33 plays an important role in allergy, as it was shown to be a mediator of inflammation and fibrotic damage [28]. Recently, Il-33 has been considered as an emerging key factor in the development of allergic diseases [29]. Notably, Il-33 expression is up-regulated in the bronchial mucosa of asthmatic patients related to disease severity [30,31]. Many authors have shown higher levels of Il-33 in asthmatic patients [32-34]. Ravanetti et al. demonstrated that Il33 was necessary to drive asthma exacerbations [35]. In addition to promoting Th2 inflammation, Zoltowska et al. suggest a role for the Il-33/ST2 pathway for the induction of peripheral inflammation and mucus production that causes airway hyper responsiveness (AHR) in the peripheral lung in a house dust mite mouse model of asthma [36].
In our study the level of Il-33 significantly increased in children who had any epitope for a dog (p = 0.002), and also significantly decreased when these children experienced any symptoms after contact with the dog (p<0.001). Particularly noteworthy are symptoms from the skin after contact with the dog - significantly affecting the increase in Il-33 concentration (p<0.001) with simultaneous strong dependence on the occurrence of dog epitopes (p<0.001). Our results suggest that higher serum Il-33 levels are not associated with asthma in dog allergic children compared to non-asthma children. Gasiuniene et al proved, that Il33 is increased in asthma patients, particularly in some phenotypes: allergic asthma and eosinophilic asthma [34]. Recently, Qiao and Chen demonstrated that the serum levels of Il-33 were significantly up-regulated in both allergic rhinitis and allergic asthma patients compared with normal individuals [37].
We found that the presence of Fel d 2 causes a reduction in the level of Il-33, the presence of Can f 3 also reduces the level of Il-33, whereas the possession of the epitope Can f 5 increases the concentration of Il-33.
Tsolakis et al demonstrated that levels of IgE to lipocalin (Fel d 4) and serum albumin (Fel d 2), but not to secretoglobin (Fel d 1) or cat extract, were independently associated with type-2 biomarkers and total IgE in young asthmatics [38]. Uriarte at al showed, that Can f 1 was associated with persistent rhinitis, Can f 2 with asthma diagnosis, Can f 3 with severity of rhinitis and asthma, and Can f 5 to both persistence and severity of rhinitis. Sensitization to several allergens in patients (1, 2, 3 or 4) was associated with persistent asthma or rhinitis, and with moderate severity. Direct contact with dogs was associated with both, persistency and severity of rhinitis [39,40]. Fel d 2 was associated with severity of rhinitis and asthma. Fel d 4 was associated with presence of asthma symptoms. Direct contact with cats was associated both with persistence and severity of rhinitis [41]. Bjerg et al. reported a higher prevalence of asthma in children cosensitized to Fel d 1 and Fel d 4 than in children sensitized to Fel d 1 alone [42]. They also saw a higher prevalence of reported asthma (55%) in patients with IgE reactivity, not only toward Fel d 1, but also toward Fel d 2 and/or Fel d 4.
Gent at al found that children sensitized and exposed to pet allergen were at significantly increased risk of wheeze (by 39% and 53% for Fel d 1 >0.12 µg/g, Can f 1>1.2 µg/g, respectively) [43]. The most frequent allergy symptoms of cat owners with a cat allergy during contact with their cats were rhinitis (sneezing, rhinorrhea, nasal congestion and itching, postnasal drip) (80.0%), followed by conjunctivitis (73.3%), cutaneous symptoms (skin itching, skin rash, angioedema, urticaria) (33.3%), and lower respiratory symptoms (sputum, dyspnea, chest discomfort and pain, wheezing) (13.3%). Dog owners with a dog allergy suffered from cutaneous symptoms more frequently than cat owners with a cat allergy (53.3% vs 33.3%), especially in terms of urticaria (35.9% vs 15.6%). Dog owners with a dog allergy also experienced lower respiratory symptoms more frequently than did cat owners with a cat allergy (33.3% vs 13.3%), especially cough (23.3% vs 6.7%) [44]. Researchers found that Il-33 was significantly up-regulated in atopic asthmatic patients compared to healthy controls [45,46]. In our study, there is no statistical relationship between the level of Il-33 and the occurrence of any symptoms after contact with the cat, both in children who had and did not have epitopes for the cat. We did not show a correlation between the level of Il-33 and the number of epitopes for the cat and the dog and the occurrence of asthma (p = 0.651) or atopic dermatitis (p = 0.274).
Our study has some limitations, including the small number of subjects. On the other hand, all patients had the same ethnicity - Caucasian, which makes it more homogeneous with a small group of patients. It also lacks control group with children who are not sensitized to cat living and not living with cat. Therefore, it is very difficult to sort out the role of a single cytokine and its relationship to the environment in a real world setting, where the environmental exposures are based on retrospective history and numerous other confounding exposures exist. In our study, all children were diagnosed with allergic rhinitis, which is why the disease was not taken into account in the statistical analysis. Käck et al stated that, sensitization to an increasing number of dog allergen components and to lipocalins is associated with dog allergy. Our study showed a directly proportional dependence between the level of Il33 and the number of IgE epitopes for the dog (p = 0.004). In addition, we have shown that when having cat and dog epitopes at the same time, there is a relationship between the level of Il-33: inversely proportional to cat epitopes (p = 0.027) and directly proportional to dog epitopes (p<0.001). We didn’t find any relationship between the level of Il-33 and the number of IgE epitopes for the cat (p = 0.814). Monosensitization to Can f 5 should not be regarded primarily as a marker for dog allergy [47]. Sensitization to Can f 5 was also recently highlighted among children with severe asthma who, compared with children with controlled asthma, had an IgE response to more than 3 animal-derived allergen molecules, of which Can f 5 was one [48,49].
Conclusion
Airway epithelial cells secrete Il-33 in response to the different allergens. Il-33 can be a useful biomarker to detect atopic asthma. In our study, we demonstrated, a positive correlation between the level of Il-33, and the number of IgE epitopes for the dog (p = 0.004). The presence of the epitope Can f 5 significantly increases the concentration of Il-33. However, we did not find any correlation between the level of Il-33 and the occurrence of cat epitopes. Interleukin-33 is a unique cytokine that plays an essential role in regulating immune responses in allergic diseases. Mechanisms of the release, expression and regulation of IL-33 in allergic diseases are not yet defined. Identification of biomarkers of atopic asthma may be useful in the future in diagnostics, determining the stage of advancement, and monitoring the treatment in various allergic diseases.
Conflict of Interest
All authors state no conflict of interests
Financial Information
This study was funded by grant 503/1-047-01/503-21- 001-18 from the Medical University of Lodz, Poland.
Trial Registration
Not applicable
Sybilski AJ, Raciborski F, Lipiec A (2015) Epidemiology of atopic dermatitis in Poland according to the Epidemiology of Allergic Disorders in Poland (ECAP) study. J Dermatol 42: 140-147.[ Ref ]
Katarzyna S, Joanna J, Daniela P, Agnieszka B (2020) Sensitization to cat and dog components and prediction of symptoms in cat-sensitized children. Research Square.[ Ref ]
Canonica GW, Ansotegui IJ, Pawankar R (2013) A WAO - ARIA - GA²LEN consensus document on molecular-based allergy diagnostics. World Allergy Organ J 6: 17.[ Ref ]
Ohman JL, Lowell FC, Bloch KJ (1974) Allergens of Mammalian Origin: III. Properties of a Major Feline Allergen. J Immunol 113: 1668-1677.[ Ref ]
Anderson MC, Baer H, Ohman JL (1985) A comparative study of the allergens of cat urine, serum, saliva, and pelt. J Allergy Clin Immunol 76: 563-569.[ Ref ]
van Milligen F, Vroom T, Aalberse R (1990) Presence of Felis domesticus allergen I in the cat’s salivary and lacrimal glands. Int Arch Allergy Appl Immunol 92: 375-378.[ Ref ]
Grönlund H, Adedoyin J, Reininger R (2008) Higher immunoglobulin E antibody levels to recombinant Fel d 1 in cat-allergic children with asthma compared with rhinoconjunctivitis. Clin Exp Allergy 38: 1275-1281.[ Ref ]
Hilger C, Kohnen M, Grigioni F (1997) Allergic cross-reactions between cat and pig serum albumin. Study at the protein and DNA levels. Allergy 52: 179-187.[ Ref ]
Smith W, Butler AJ, Hazell LA (2004) Fel d 4, a cat lipocalin allergen. Clin Exp Allergy 34: 1732-1738.[ Ref ]
Hilger C, Swiontek K, Arumugam K (2012) Identification of a new major dog allergen highly cross-reactive with Fel d 4 in a population of cat- and dog-sensitized patients. J Allergy Clin Immunol 129: 1149-1151.e1142.[ Ref ]
Ichikawa K, Vailes LD, Pomés A (2001) Molecular cloning, expression and modelling of cat allergen, cystatin (Fel d 3), a cysteine protease inhibitor. Clin Exp Allergy 31: 1279-1286. [ Ref ]
Adédoyin J, Grönlund H, Öman H (2007) Cat IgA, representative of new carbohydrate cross-reactive allergens. J Allergy Clin Immunol 119: 640-645. [ Ref ]
Smith W, O’Neil SE, Hales BJ (2011) Two newly identified cat allergens: the von ebner gland protein Fel d 7 and the latherin-like protein Fel d 8. Int Arch Allergy Immunol 156: 159-170.[ Ref ]
Mattsson L, Lundgren T, Olsson P (2010) Molecular and immunological characterization of Can f 4: a dog dander allergen cross-reactive with a 23 kDa odorant-binding protein in cow dander. Clin Exp Allergy 40:1276-1287. [ Ref ]
Mattsson L, Lundgren T, Everberg H (2009) Prostatic kallikrein: a new major dog allergen. J Allergy Clin Immunol 123: 362-368.[ Ref ]
Khurana T, Newman-Lindsay S, Young PR (2016) The NPC2 protein: A novel dog allergen. Ann Allergy Asthma Immunol 116: 440-446 e442.[ Ref ]
Virtanen T, Kinnunen T, Rytkonen-Nissinen M (2012) Mammalian lipocalin allergens–insights into their enigmatic allergenicity. Clin Exp Allergy 42: 494-504.[ Ref ]
Hilger C, Kuehn A, Hentges F (2012) Animal lipocalin allergens. Curr Allergy Asthma Rep 12: 438-447. [ Ref ]
Takatori H, Makita S, Ito T (2018) Regulatory Mechanisms of IL-33- ST2-Mediated Allergic Inflammation. Front Immunol 9: 2004.[ Ref ]
Liccardi G, Triggiani M, Piccolo A (2016) Sensitization to Common and Uncommon Pets or Other Furry Animals: Which May Be Common Mechanisms? Transl Med UniSa 14: 9-14.[ Ref ]
Ding W, Zou GL, Zhang W (2018) Interleukin-33: Its Emerging Role in Allergic Diseases. Molecules 23: 1665.[ Ref ]
Soyka MB, Holzmann D, Basinski TM (2015) The Induction of IL-33 in the Sinus Epithelium and Its Influence on T-Helper Cell Responses. PLoS ONE 10: e0123163.[ Ref ]
Liao B, Cao PP, Zeng M (2015) Interaction of thymic stromal lymphopoietin, IL-33, and their receptors in epithelial cells in eosinophilic chronic rhinosinusitis with nasal polyps. Allergy 70:1169-1180.[ Ref ]
Ozturan A, Eyigor H, Eyigor M (2017) The role of IL-25 and IL-33 in chronic rhinosinusitis with or without nasal polyps. Eur Arch Otorhinolaryngol 274: 283-288. [ Ref ]
Becker AB, Abrams EM (2017) Asthma guidelines: the Global Initiative for Asthma in relation to national guidelines. Curr Opin Allergy Clin Immunol 17: 99-103.[ Ref ]
Eichenfield LF, Hanifin JM, Luger TA (2003) Consensus conference on pediatric atopic dermatitis. J Am Acad Dermatol 49: 1088-1095.[ Ref ]
Bousquet J, Schünemann HJ, Samolinski B (2012) World Health Organization Collaborating Center for Asthma and Rhinitis. Allergic Rhinitis and its impact on asthma (ARIA): achievements in 10 years and future needs. J Allergy Clin Immunol 130: 1049-1062.[ Ref ]
Guo Z, Wu J, Zhao J (2014) IL-33 promotes airway remodeling and is a marker of asthma disease severity. J. Asthma 51: 863-869.[ Ref ]
Saluja R, Khan M, Church MK (2015) The role of IL-33 and mast cells in allergy and inflammation. Clin Transl Allergy 5: 33.[ Ref ]
Bianchetti L, Marini MA, Isgrò M (2012) IL-33 promotes the migration and proliferation of circulating fibrocytes from patients with allergenexacerbated asthma. Biochem Biophys Res Commun 426: 116-121.[ Ref ]
Li Y, Wang W, Lv Z (2018) Elevated Expression of IL-33 and TSLP in the Airways of Human Asthmatics In Vivo: A Potential Biomarker of Severe Refractory Disease. Immunol 200: 2253-2262. [ Ref ]
Momen T, Ahanchian H, Reisi M (2017) Comparison of Interleukin-33 Serum Levels in Asthmatic Patients with a Control Group and Relation with the Severity of the Disease. Int J Prev Med 8: 65. [ Ref ]
Poulsen NN, Bjerregaard A, Khoo SK (2018) Airway Interleukin-33 and type 2 cytokines in adult patients with acute asthma. Respir Med 140:50-56. [ Ref ]
Gasiuniene E, Janulaityte I, Zemeckiene Z (2018) Elevated levels of interleukin-33 are associated with allergic and eosinophilic asthma. Scand J Immunol 89: e12724.[ Ref ]
Ravanetti L, Dijkhuis A, Dekker T (2018) IL-33 drives influenzainduced asthma exacerbations by halting innate and adaptive antiviral immunity. J Allergy Clin Immunol 143: 1355-1370.e16.[ Ref ]
Zoltowska AM, Lei Y, Fuchs B (2016) The interleukin-33 receptor ST2 is important for the development of peripheral airway hyperresponsiveness and inflammation in a house dust mite mouse model of asthma. Clin Exp Allergy 46: 479-490.[ Ref ]
Qiao Y, Chen J (2018) Serum levels of IL-31, IL-33 and ST2 in allergic rhinitis of children in China. Cell Mol Biol (Noisy-le-grand) 64: 52-55.[ Ref ]
Tsolakis N, Malinovschi A, Nordvall L (2018) Sensitization to minor cat allergen components is associated with type-2 biomarkers in young asthmatics. Clin Exp Allergy 48: 1186-1194.[ Ref ]
Uriarte SA, Sastre J (2016) Clinical relevance of molecular diagnosis in pet allergy. Allergy 71: 1066-1068. [ Ref ]
Uriarte SO, Sastre J (2014) Clinical impact of molecular diagnosis in dog allergy. Clin Transl Allergy 4(Suppl 2): P52.[ Ref ]
Uriarte SO, Sastre J (2014) Clinical impact of molecular diagnosis in cat allergy. Clin Transl Allergy 4(Suppl 2): P51.[ Ref ]
Bjerg A, Winberg A, Berthold M (2015) A population-based study of animal component sensitization, asthma and rhinitis in schoolchildren. Pediatr Allergy Immunol 26: 557-563.[ Ref ]
Gent JF, Kezik JM, Hill ME (2012) Household mold and dust allergens: exposure, sensitization and childhood asthma morbidity. Environ Res 118: 86-93.[ Ref ]
Yang MS, Lee SP, Kwon YJ (2018) Dog and Cat Allergies and Allergen Avoidance Measures in Korean Adult Pet Owners Who Participated in a Pet Exhibition. Allergy Asthma Immunol Res 10: 155-164.[ Ref ]
Jackson DJ, Makrinioti H, Rana BM (2014) IL-33-Dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am J Respir Crit Care Med 190: 1373-1382.[ Ref ]
Bhowmik M, Majumdar S, Dasgupta A (2019) Pilot-Scale Study Of Human Plasma Proteomics Identifies ApoE And IL33 As Markers In Atopic Asthma. J Asthma Allergy 12: 273-283.[ Ref ]
Käck U, Asarnoj A, Grönlund H (2018) Molecular allergy diagnostics refine characterization of children sensitized to dog dander. J Allergy Clin Immunol 142: 1113-1120.e9.[ Ref ]
Nordlund B, Konradsen JR, Kull I (2012) IgE antibodies to animalderived lipocalin, kallikrein and secretoglobin are markers of bronchial inflammation in severe childhood asthma. Allergy 67: 661-669.[ Ref ]