Journal Name: Journal of Pediatrics and Infants
Article Type: Short communication
Received date: 26 November, 2020
Accepted date: 18 December, 2020
Published date: 23 December, 2020
Citation:Devaskar UP, Waghmare S, Kharche A, Kalane SU, Haridas V, et al (2020) KIMIE: New Human Breast Milk Pasteurizer-Fully Automated, User Friendly, CostEffective Device for Universal Application. J Pediat Infants Vol: 3, Issu: 2 (30-37).
Copyright: © 2020 Devaskar UP et al. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Natural mother’s breast milk (MBM) is the best food for all newborns especially the preterm. However, when natural MBM is not available or insufficient donor breast milk (DBM) is the second-best option. Sterifeed or HSC human milk pasteurizers have been commonly used for several decades. While these devices have served the purpose, they are relatively large, expensive, require special electrical and water connections, need a large amount of water which is not recycled and an ongoing supply of disposable plastic bottles. In addition, the use of these machines requires special training. Here we describe the development of a compact, automated; user-friendly human breast milk pasteurizer (HBMP) named Kimie capable of pasteurizing small volumes of DBM. This device does not require special water plumbing, recycles water, is inexpensive and does not require FDA approval.
Keywords: Human milk bank, Pasteurizer, Breast milk, Neonate.
Abbreviations
AGA: Appropriate for gestational age,
BM: Breast milk,
DBM: Donor breast milk,
DBMB: Donor breast milk bank,
EBM: Expressed breast milk,
HTLV: Human T-cell leukemia virus,
HBMP: Human breast milk pasteurizer,
IUGR: Intrauterine growth restriction,
MBM: Mother’s breast milk,
NEC: Necrotizing enterocolitis,
NICU: Neonatal intensive care unit,
PDBM: Pasteurized donor breast milk,
TPN: Total parenteral nutrition
Introduction
Natural mother’s breast milk (MBM) is the best of all feeding options for all newborns especially the preterm (1-6). However, natural MBM is not always available, can be insufficient to provide an adequate amount (soon after delivery, maternal refusal or death) or is contraindicated (maternal illness, use of illicit drugs or medications, or un-satisfactory collection practices). Under these circumstances pasteurized donor breast milk (PDBM) is considered the second-best option as compared to the formulas derived from cow’s milk [1-7]. Therefore, PDBM is commonly used in Neonatal Intensive Care Units (NICUs) worldwide. Many bodies like WHO, the United States Breastfeeding Committee, the American Academy of Pediatrics, European Society for Pediatric Gastroenterology, Hepatology and Nutrition, and the Indian Academy of Pediatrics have emphasized the importance of donor breast milk (DBM) and acknowledged that donor breast milk banks (DBMB) play an important role in the well-being of preterm babies [1,8-14].
Traditional DBMB are operated independently and are not hospital-based. They sell PDBM to NICUs within their region [15-17]. However, there are an increasing number of inhospital DBMB [1,18,19]. In the traditional DBMB, Sterifeed (or) HSC HBMP have been used for many decades. During the process of Holder pasteurization, most of the viruses and bacteria are killed while preserving most of the nutritional and immunologic properties of MBM [1,15,20-23]. These devices are large, expensive, require special electrical and water connections, need a large amount of water which is not recycled and an ongoing supply of disposable plastic bottles. In addition, use of these devices requires special training. To be cost effective they require a large volume of DBM to be pasteurized during each cycle. Therefore, the use of these devices is limited to DBMB in developed countries or larger hospitals in big cities in countries like India. Here we describe the development of a compact, automated, userfriendly HBMP capable of pasteurizing small volumes of DBM. This device does not require special water plumbing, recycles water and is inexpensive. It is designed mostly for the in-hospital installed DBMB.
Materials and Methods
In December, 2017 health care providers and both engineers met and decided to develop a HBMP using the principle of the widely used Holder technology (Figure 1).
Figure 1: Graphical presentation of Holder Technology.
It was discovered that current pasteurizers are connected to a water source for filling the chamber and a separate plumbing is needed to drain the water. It uses a submersible water heater of very high wattage. To distribute heat evenly, it incorporates a water stirrer. Use of a submersible water heater is concerning due to a risk of electrical shock. In addition, the waste of large amount, ~ 80 liters, of water after each cycle was considered problematic.
Various options to manufacture a compact, portable, user-and eco-friendly, cost-effective device using modern technology were discussed. It was decided to use two separate water tanks, one for heating and one for cooling. Two different water pumps were connected to the respective tanks. In this closed loop system, each pump propels water in the chamber which warms or cools DBM and water returns to its respective tank. The capacity of heating and cooling tanks was arbitrarily decided to be 1,750 and 2,750 ml respectively. Thus only 4,500 ml of water would be used and recycled. Other discussions included: the technical and tubing design, the method of water circulation around the milk containers, the components required and their placement, the type of water pumps for heating and cooling, the required flow rate, its durability, the type of heaters and their wattage, the type of cooling mechanism (chiller) and their capacity, the size and shape of DBM containers. To be eco-friendly, for easy cleaning and to be cost-effective, it was decided to use grade 304 stainless-steel cylinders instead of the disposable plastic bottles [24]. Eventually we developed a closed loop system for circulating hot and cold water around the milk containers by using submersible water pumps, a sandwich heater mechanism, solenoid valves, a water tight steel container and a microprocessor- based temperature indicator and controller with a timer mechanism. The electronic temperature indicator and controller were used with a sensor to measure the milk temperature in real time. Technology to separately display the milk temperature and the duration of the cycle in real time in digital form were also devised.
In this device, Perplex, a better material compared to plastic, is used. The pasteurization chamber is designed in molded plastic with a cavity in between the walls of the chamber to avoid energy losses. This air gap acts as thermal protection ensuring that heating or cooling energy is conserved. The water inlet is on one side while the water outlet is on the opposite to ensure laminar flow. An auto-drain facility utilized after hold or cool mode and completion of cycle were implemented. The main chamber is fitted with a separate water draining facility at the bottom of the chamber. A separate small compartment is provided to collect and then drain the over flowed water. This chamber has a separate outlet to drain hot or cold water to their respective reservoirs during the respective cycle. The pasteurization chamber is fitted with a properly fitting lid on the top.
A stainless-steel cage holds six cylinders, one for water and the other for DBM. The cage is provided with a water temperature sensor. The height of the cage is maintained from 0.3 to 1 cm ensuring that the cylinders are submerged to the desired level during both cycles. It is fitted with a special mechanism that ensures that the cylinders do not float in water even if the milk volume is small. This ensures that the cylinders are fully submerged in water to 2.5 cm during the entire cycle. A separate stainless-steel cylinder numbered one, to measure the temperature of the water using a sensor in real time, was built. In addition, five different stainlesssteel cylinders, numbered two to five, with a maximum capacity of 100 ml each, were introduced. Thus, a maximum of 500 ml of DBM can be pasteurized during each cycle. Special ring is used to ensure that water does not leak into the cylinder (Figures 2 and 3).
Figure 2: Kimie: The Bird’s Eye Views.
Figure 3: Kimie: The Bird’s Eye Views.
A water recirculating pump (model BLDC, Iwaki, Japan) which can withstand temperature of 80ºC and has a longer shelf life is used. It can also operate on 24 Volts DC, has a potentiometer to adjust the flow of water electronically, and comes with a lifetime warranty of 25,000 hours. Since we needed lesser flow, we incorporated a flow control valve towards the heater and the chiller. A water reservoir has a separate drain valve to drain the water after each cycle. It also has an overflow outlet to ensure that the water level in the tank is always maintained at the desired level. The reservoir also has a water inlet to refill the tank. Two sensors are placed in the pasteurization chamber to measure the temperature of the circulating water around the milk containers and the temperature of the water inside the cylinder. The level of water is maintained in the chamber 0.2 cm above the milk level to ensure effective treatment. Once the water level reaches 0.2 cm above the milk container, it starts flowing above the specially constructed restriction wall. It is then collected in the retraction chamber from where it is drained through a sol valve and is returned back to the hot water reservoir. Seven sol valves are used in the system, the diameter varying from 0.8 to 1.2 cm.
A separate flat brass heater of 600 watts is placed in an aluminum enclosure. It has 2 parts: male and female. Special grooves are made in the enclosures with a width of 6 mm and a depth of 4 mm, through which the water passes. This is the female compartment. The heater is placed pressed on both sides of the enclosures for even heating of water. Heavy insulation is used to avoid radiative heat loss. The male part is then fixed on the female part.
After the hold cycle is completed after 30 minutes, the microcontroller indicates: DRN 1. Simultaneously the drain valve for the heating mechanism activates and drains the hot water in the compartment within 20 to 30 seconds. Then drain valve 1 is switched off. The hot water tank consists of one water inlet, one water outlet, one overflow outlet and one drain outlet. A separate filter is incorporated at the water inlet to ensure that foreign particles do not enter the system. The hot water tank has a separate outlet that supplies water to the pump which is pumped in the system and then returns back to the reservoir tank via a separate inlet.
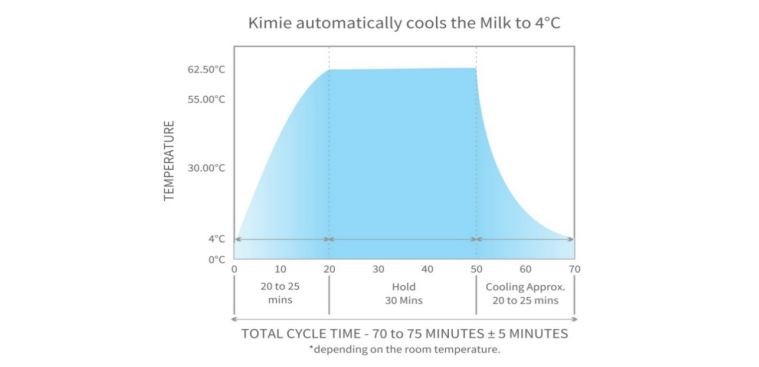
We found a chiller to decrease the milk temperature to 4ºC within 25 minutes. A separate sensor mechanism was instituted for more accuracy and faster warming. When the system is switched on the heating cycle starts. Simultaneously, the cooling system is also switched on. The sol valve S2 and S5 are switched on. The pump sucks the water from the reservoir tank and pushes it to the chiller via the sol valve S2 and then returns to the tank via solve valve S5. A separate water sensor is placed in the reservoir tank that shuts off the chiller when the tank water temperature reaches 1.5ºC. Once the temperature rises to 2.5ºC, the chiller is switched on again. This process continues through the entire cycle ensuring that cold water is readily available when the cooling process starts. A specially developed chiller is able to cool the water from room temperature to 1.5ºC in 30 to 35 minutes. When the cycle is over, the microcontroller indicates DRN 2 and the drain valve is activated to drain the cold water within 20 to 30 seconds. After 30 seconds, the drain valve number 2 is switched off.
A waterproof stainless-steel sensor is fitted inside the water cylinder number one. It measures the water temperature in real time and servo controls the heater output via a microprocessor. If the water temperature exceeds 63.5ºC in heating mode, it shuts off the heater. During the hold mode the water temperature sensor cuts off the heater if the water temperature exceeds 63.5ºC. This dual control ensures that the milk temperature never exceeds 63.5ºC. Another sensor is fitted in the pasteurization chamber that measures the water temperature. This water sensor is fitted to the steel cage that holds the milk cylinders. Another sensor measures the temperature in the cooling system.
The LED display indicates the elapsed time and temperature of water (ºC) in real time.
We performed a comparative study for bacteriologic testing of DBM before and after pasteurization using HSC (control group) or Kimie (study group). This study was approved by the IRB at DMH. Prior to pasteurization, 1-3 ml of DBM in a sterile container (n=100) was sent to the microbiology laboratory. The remaining DBM was divided into two equal portions. The volume ranged from 20 to 60 ml. They were pasteurized separately using HSC or Kimie. Subsequently, 1-3 ml of PDBM subjected to bacteriological testing using sheep blood Agar plates or McKonkey medium [25]. Incubation was performed for 48-72 hours at 37ºC [25]. A qualitative and semi-quantitative analysis of the bacterial growth was performed. We did not attempt to sub-classify the species of bacterial growth.
Results
We performed trials by placing six steel containers filled with water and submerged two sensors in two different containers to measure and compare the temperatures during the cycle. Two different temperature indicators and controllers were used. All the containers depicted the same temperature during the entire cycle. More than 100 satisfactory trials were conducted to verify and ensure the accuracy of the milk temperature during the hold or cooling mode. We placed two sensors in two different milk containers which were in opposite direction. We noted the temperature indicated by the master sensor and the temperature displayed by the second (slave) sensor. The slave sensor showed a difference of 1.5ºC before the start of the cycle and continued to show that temperature during the entire cycle. We recorded the difference. In cooling mode the same things occurred. This confirmed that the heat transfer during the entire pasteurization process was equal and effective in all the six compartments of the pasteurization chambers. The average difference shown by the temperature sensors was 1.4 to 1.6ºC and was constant during all the trials.
All of the DBM samples before pasteurization grew Gram Positive (n=34, colony count 3 x 102 to 1 x 105), Gram Negative (n=36, colony count 1 x 102 to 1 x 105) or both (n=30) bacteria. However, all of the DBM samples except one were sterile in both the groups after pasteurization. The colony count did decrease in both the positive growth samples suggesting partial pasteurization. We concluded that Kimie was equally effective in killing bacteria when compared to HSC.
After washing the cylinders and the plastic lids with a detergent, rinse them thoroughly with water to remove the detergent. The cylinder and the plastic lids can be sterilized by using any suitable sterilization method for stainless-steel and plastic. These include but are not limited to Sterrad hydrogen peroxide plasma based low temperature or any other low temperature gas sterilization system. One may also use a dishwasher or submerge the cylinders and the plastic lids in a large container with boiling hot water for ten minutes. Then remove them carefully and place them on a clean surface until completely dry.
Kimie has received the European Certificate of compliance (no 1810170911101) for a Human Milk Pasteurizer and met Standards EN 6060-1, EN 60601-1-2, and EN 60601-1-6 to demonstrate conformity. In the USA, any BM pasteurizer does not require federal drug administration approval since it is considered a catering device. Kimie was imported to the USA from India under commodity code number 84198998.
We have developed an operating instructions manual for an easy use of Kimie (not included).
Discussion
We describe the development of a portable, fully automated, user friendly, cost effective HBM which uses modern technology, requires less space, no special plumbing, and can recycle water. Digital screens display time and temperature in real time. Heating or cooling is controlled by intelligent microcontrollers ensuring temperature accuracy. The device is capable of pasteurizing 10-500 ml of EBM. It has been used successfully in four hospital-installed DBMB in India for more than a year and a half and at Centinela Hospital Medical Centre, Inglewood, California for more than two months.
In the food industry worldwide, the regulating agencies prefer to use stainless-steel for heating, cooling or drying food because it prevents any chemical reaction between the food and the container [24]. The Food and Drug Association’s rule states that materials used in the construction of utensils and food contact surfaces may not allow the migration of deleterious substances or impart color, odor, or tastes to food. Such material needs to be safe, durable, corrosionresistant to many chemicals, non-absorbent, sufficient in weight and thickness to withstand repeated washing, have a smooth, easy-to-clean surface, resistant to pitting, chipping, crazing, scratching and scoring. Therefore, we chose stainless-steel grade 304 rather than plastic bottles. This type of stainless steel is most commonly used as alloy in a variety of industries since it can resist corrosion caused by several chemicals and can be electro-polished to a smooth, shiny and easy-to-clean surface.
In this device, the water level during heating or cooling mode is above the milk level ensuring BM is evenly treated with the provided energy. During heating or cooling it is an unsteady state of energy transfer meaning the temperature difference between the DBM and water is high. However, as the temperature difference between the water and DBM reduces, the energy transfer is slow. Therefore, as per the law of thermodynamics, it is called an unsteady state. We conducted several experiments to determine the optimum rate of heat transfer so that the desired milk temperature can be reached in the shortest time, which will be cost effective regarding power consumption and time. To accomplish this goal, we varied the flow of water entering the pasteurization chamber, its effects on warming and cooling and the time taken. Then we varied the heater wattage and heater output power via micro-controllers to achieve fast warming without over shooting it. In these trials we ensured that the milk temperature never exceeded 63ºC. Lastly, we invented a sandwich heating mechanism which ensures that each drop of water entering the heating body is evenly heated to the same temperature during the entire travel time. We manufactured the heater body in stainless-steel for better heat transfer as compared to aluminum.
There is always a gap between the milk and the lid of the milk container. During the heating process, heat transfer will only happen when there is a temperature difference between milk and the air. As the milk temperature starts rising, the air temperature will also start rising due to convective heat transfer. As the temperature difference is high, the rate of heat transfer will be also very high, but as the temperature difference between air and milk reduces, there will be no heat transfer to the air. This state is defined as equilibrium.
We measured the milk and air temperature using two different probes and the temperature difference was recorded. When the milk temperature was 62.7ºC, the air temperature was 62.3ºC. This did not change even when we increased the time of heating because the system reached the stage of equilibrium. In the heating mode equilibrium is achieved in 25 minutes.
There are concerns with fully submerging plastic bottle containers under water during the heating. As used in other pasteurizers. plastic is an insulation material therefore heat conduction is poor. As a result, it is necessary to stir the water for better heat transfer. In Kimie, stirring the milk is not necessary because the conductive property of steel is 100 times faster than plastic [24]. Submerging the plastic bottles under water increases the time for heating, consumes more energy and needs more water and is not Eco friendly. Even if recycled, plastic bottles will need to be discarded after a few cycles. Stainless-steel containers can be reused indefinitely.
Debate continues regarding early postnatal readiness for enteral feeding in premature appropriate for gestational age (AGA) or intrauterine growth restricted (IUGR) neonates [25- 38]. Early enteral feeding has been associated with improved survival and postnatal growth, decreased incidence of sepsis and necrotizing enterocolitis (NEC) and fewer days of total parenteral nutrition [25,26,29,34-36]. Therefore, there is an increasing trend to initiate at least minimal enteral nutrition (trophic feedings) as early as 6 hours of life and also for early aggresive enteral nutrition [25-29]. A baby’s own mother’s expressed breast milk (EBM) including the colostrum, is the best feeding option. The overarching goal is to reach full enteral EBM feeding in the shortest possible time [25- 38]. However, when mother’s EBM milk is not available, insufficient or not suitable, supplementation with PDBM or specially designed preterm formulas is a common practice [1-6]. PDBM is considered to be a better option than the preterm formula [1-6].
Detailed operation of traditional DBMB has been described [15-17,39-49]. These banks rely on a donor breast-feeding population to ensure an adequate supply of DBM [15-17,39-45]. In general, donor mothers have delivered a baby at full-term gestation and have been lactating for several weeks or months [15-17,36-39]. These women, under prescription from a physician, donate their extra BM for vulnerable babies [15-17]. These mothers are screened for medical and lifestyle risk factors, serum for syphilis, HIV, Hepatitis B and C, and human T-cell leukemia virus (HTLV) [15-17]. Staff members of the traditional DBMB instruct women for proper collection and storage of milk at home in hygienic conditions [15-17]. Subsequently, they are instructed to transfer stored frozen DBM to the DBMB in a suitable insulated container on ice or ice packs. In this model, it is assumed that the donor mother has a refrigerator at home which they may not. At these banks, collected DBM milk is stored frozen until pasteurized and then frozen again until shipped to the regional NICU. DBM is frozen and thawed up to three times before it is consumed by the baby. In general, DBM from four to six mothers is pooled for pasteurization. On the other hand, in the in-hospital installed DBMB setting, a physician, nurse or both approach all mothers who deliver premature babies in the immediate post-partum period. Information regarding their medical and life style risk factors, and prenatal serum syphilis, HIV, Hepatitis B and C, HTLV is readily available from the prenatal records even in developing countries like India. No additional testing or an order for donation by a physician is needed. The NICU staff educates these women regarding the need for expressing milk, the importance of breast hygiene, proper collection and storage of EBM. Several times BM is expressed in the hospital under supervision of a nurse or a lactation specialist. These mothers are requested, when needed, to express milk more than what their baby requires, The extra fresh EBM can be easily pasteurized shortly and stored at 4ºC in small aliquots to be used within the next 24 hours or stored frozen at -18ºC for use after 24 hours.
In summary, we have developed a human breast milk pasteurizer better suited for in-hospital installed DBMB that should help in improving the health of premature babies worldwide.
Acknowledgement
We would like to thank the entire NICU nursing staff at Deenanath Mangeshkar Hospital for their help. We are gratefulk to all the mothers’ who provided their breast milk.
Authors’ Contribution
Initially, Dr. Uday Devaskar, Dr. Shilpa Kalane and Mrs. Vishakha Haridas had several discussions regarding the limitations of existing human breast milk pasteurizers. It was concluded there was a need for a new pasteurizer that would be user friendly, cost effective and capable of pasteurizing relatively small volume of milk. Dr. Devaskar developed the design of such a pasteurizer. Then they met with Mr. Sudhir Waghmare and Mr. Ashay Kharche on several times. Mr. Waghmare and Mr. Kharche developed first and then the second prototype of the pasteurizer named Kimie. Their design, mode of operation, utility and limitations were discussed by the group which led to the genesis of the present (third) prototype. Dr. Kalane obtained the IRB approval while Mrs. Haridas obtained consent from the lactating mothers to perform bacteriologic testing of the expressed breast milk before and after pasteurizer using Kimie or HSC. Mrs. Sampada Patwardhan performed the bacteriologic cultures and analyzed all the results. While Mr. Waghmare and Mr. Kharche wrote the engineering part, Dr. Devaskar and Dr. Kalane have written the medical portion.
Conflict of Interests
There is no conflict of interest.
References
Picaud JC, Buffin R (2017) Human Milk-Treatment and Quality of Banked Human Milk. Clin Perinatol 44: 95-119. [ Ref ]
Quigley M, McGuire W (2014) Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev 22: CD002971. [ Ref ]
Section on breastfeeding (2012) Breastfeeding and the Use of Human Milk. Pediatrics 129: e827-e841. [ Ref ]
Arslanoglu S, Corpeleijn W, Moro G, ESPGHAN Committee on Nutrition, et al. (2013) Donor human milk for preterm infants: current evidence and research directions. J Pediatr Gastroenterol Nutr 57: 535-542. [ Ref ]
Agostoni C, Buonocore G, Carnielli VP, ESPGHAN Committee on Nutrition, et al. (2010) Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr 50: 85-91. [ Ref ]
Arslanoglu S, Moro GE, Bellu R, De Nisi G, Tonetto P, et al. (2013) Presence of human milk bank is associated with elevated rate of exclusive breastfeeding in VLBW infants. J Perinat Med 41: 129-131. [ Ref ]
Miller J, Tonkin E, Damarell RA, McPhee AJ, Suganuma M, et al. (2018) A systematic review and meta-analysis of human milk feeding and morbidity in very low birth weight infants. Nutrients 10: 707. [ Ref ]
World Health Organization. Breastfeeding. [ Ref ]
United States Breastfeeding Committee. USBC: a brief history. [ Ref ]
American Academy of Pediatrics (2012) Breast feeding and the use of human milk. Pediatrics 129: e827-e841. [ Ref ]
American Academy of Pediatrics Committee on Nutrition (2005) Breastfeeding and the use of human milk. Pediatrics 115: 496-506. [ Ref ]
Sachdeva R, Mondkar J, Shanbhag S, Sinha M, Khan A, et al. (2019) A landscape analysis of human milk banks in India. Indian Pediatr 56:663-668. [ Ref ]
Bharadva K, Tiwari S, Mishra S, Mukhopadhyay K, Yadav B, et al. (2014) Human Milk Banking Guidelines. Indian Pediatr 51: 469-474. [ Ref ]
Willamson S, Hewitt JH, Finucane E (1978) Organization of bank of raw and pasteurized human milk for NICU care. British Medical journal 1: 393-396. [ Ref ]
Human Milk Banking Association of North America (2018) Guidelines for the Establishment and Operation of a Donor Human Milk Bank Human. Milk Banking Association of North America, Fort Worth, TX. [ Ref ]
.Jones F (2019) Best Practice for Expressing, Storing and Handling Human Milk in Hospitals, Homes, and Child Care Settings. Human Milk Banking Association of North America, Fort Worth, TX. [ Ref ]
Human Milk Bank Association of North America (2013) Guidelines for the establishment and operation of a donor Human Milk Bank. [ Ref ]
Hurst NM, Myatt A, Schanler RJ (1998) Growth and development of a hospital-based lactation program and mother’s own milk bank. J Obstet Gynecol Neonatal Nurs 27: 503-510. [ Ref ]
Mizuno K, Sakurai M, Itabashi K (2015) Necessity of human milk banking in Japan: questionnaire survey of neonatologists. Pediatr Int 57: 639-644. [ Ref ]
Yuen JW, Lake AY, Gohel MDL (2012) Nutritional and immunological characteristics of fresh and refrigerated stored human milk in Hong Kong: a pilot study. Clinica Chimica Acta 413: 1549-1554. [ Ref ]
Takci S, Gulmez D, Yigit S, Dogan O, Dik K, et al. (2012) Effects of freezing on the bactericidal activity of human milk. J Pediatr Gastroenterol Nutr 55: 146-149. [ Ref ]
Chang JC, Chen CH, Fang LJ, Tsai CR, Chang YC, et al. (2013) Influence of prolonged storage process, pasteurization, and heat treatment on biologically-active human milk proteins. Pediatr Neonatal 54: 360-366. [ Ref ]
Raoff NA, Adamkin DH, Radmacher PG, Telang S (2016) Comparison of lactoferrin activity in fresh and stored milk. J Perinatal 36: 207-209. [ Ref ]
www.marlinware.com [ Ref ]
.Modi M, Ramji S, Jain A, Kumar P, Gupta N (2019) Early Aggressive Enteral Feeding in Neonates Weighing 750–1250 Grams: A Randomized Controlled Trial. Indian Pediatrics 56: 294-298. [ Ref ]
Rayyis SF, Ambalavanan N, Wright L, Carlo WA (1999) Randomized trial of “slow” versus “fast” feed advancements on the incidence of necrotizing enterocolitis in very low birth weight. J Pediatr 134: 293-297. [ Ref ]
Klotz D, Jansen S, Gebauer C, Fuchs H (2018) Handling of breast milk by neonatal units: large differences in current practices and beliefs. Front Pediatr 6: 235.[ Ref ]
Lenhartova N, Matasova K, Lasabova Z, Javorka K, Calkovska A (2017) Impact of early aggressive nutrition on retinal development in premature infants. Physio Res 66: S215-S226. [ Ref ]
Tewari VV, Dubey SK, Kumar R, Vardhan S, Sreedhar CM, et al (2018) Early versus Late Enteral Feeding in Preterm Intrauterine Growth Restricted Neonates with Antenatal Doppler Abnormalities: An OpenLabel Randomized Trial. Journal of Tropical Pediatrics 64: 4-14. [ Ref ]
Dutta S, Singh B, Chessell L, Wilson J, Janes M, et al. (2015) Guidelines for feeding very low birth weight infants. Nutrients 7: 423-442. [ Ref ]
Meier PP, Engstrom JL, Patel AL, Jegier BJ, Bruns NE (2010) Improving the use of human milk during and after the NICU stay. Clin Perinatol 37: 217-245. [ Ref ]
Rodriguez NA, Meier PP, Groer MW (2009) Oropharyngeal administration of colostrum to extremely low birth weight infants: theoretical perspectives. J Perinatol 29: 1-7. [ Ref ]
Rodriguez NA, Meier PP, Groer MW (2010) A pilot study of the oropharyngeal administration of own mother’s colostrum to extremely low birth weight infants. Adv Neonatal Care 10: 206-212. [ Ref ]
Garofalo NA, Caplan MS (2019) Oropharyngeal Mother’s Milk: State of the Science and Influence on Necrotizing Enterocolitis. Clin Perinatol 46: 77-88. [ Ref ]
Flidel-Rimon O, Friedman S, Lev E (2004) Early enteral feeding and nosocomial sepsis in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed 89: F289-F292. [ Ref ]
Hamilton E, Massey C, Ross J, Taylor S (2014) Early enteral feeding in very low birth weight infants. Early Hum Dev 90: 227-230. [ Ref ]
Rouwet E, Heineman E, Buurman W, Riet G, Ramsay G, et al. (2002) Intestinal Permeability and Carrier-Mediated Monosaccharide Absorption in Preterm Neonates during the Early Postnatal Period. Pediatr Res 51: 64-70. [ Ref ]
McCallie K, Lee H, Mayer O, Cohen RS, Hintz SR, et al. (2011) Improved outcomes with a standardized feeding protocol for very low birth weight infants. J Perinatol 31: S61-S67. [ Ref ]
Human Milk Banking Association of South Africa. Guidelines for the Operation of a Donor Human Milk Bank in South Africa: best practice for the collection, storage and handling of human milk. [ Ref ]
Hartmann BT, Pang WW, Keil AD, Hartmann PE, Simmer K, et al. (2007) Australian Neonatal Clinical Care Unit. Best practice guidelines for the operation of a donor human milk bank in an Australian NICU. Early Hum Dev 83: 667-673. [ Ref ]
French Human Milk Bank Association. The good practice rules for the collection, preparation, qualification, treatment, storage, distribution and dispensing on medical prescription of human milk by the milk banks. [ Ref ]
Grovslien AH, Gronn M (2009) Donor milk banking and breastfeeding in Norway. J Hum Lact 25: 206-210. [ Ref ]
National Institute for Health and Clinical Excellence. Donor breast milk banks: the operation of donor milk bank services. [ Ref ]
Arslanoglu S, Bertino E, Tonetto P, et al. (2010) Guidelines for the establishment and operation of a donor human milk bank. J Matern Fetal Neonatal Med 23: 1-20. [ Ref ]
Frischknecht K, Walchli C, Annen C (2010) Recommandations pour l’organisation et le fonctionnement d’une banque de lait en Suisse. Paediatrica 21: 24-28. [ Ref ]
Milknet. Guidelines for use of human milk and milk handling in Sweden 2011. [ Ref ]
Hartmann BT (2017) Ensuring Safety in Donor Human Milk Banking in Neonatal Intensive Care. Clin Perinatol 44: 131-149. [ Ref ]
Landers S, Hartmann BT (2013) Donor human milk banking and the emergence of milk sharing. Pediatr Clin North Am 60: 247-260. [ Ref ]
Hartmann BT, Christen L (2013) Donor human milk banking in neonatal intensive care. Nutrition for the Preterm Neonate: A Clinical Perspective. Springer. [ Ref ]
Lonnerdal B (2017) Bioactive proteins in human milk-potential benefits for preterm infants. Clin Perinatol 44: 179-191. [ Ref ]
Lonnerdal B (2003) Nutritional and phyiologic significance of human milk proteins. Am J Clin Nutr 77: 1537S-1543S. [ Ref ]
Lonnerdal B (2010) Bioactive proteins in human milk: Mechanisms of action. J Pediatr 156 (suppl2): S26-S30. [ Ref ]
Molinari CE, Casadio YS, Hartmann BT, Livk A, Bringans S, et al. (2012) Proteome mapping of human skim milk proteins in term and preterm milk. J Proteome Res 11: 1696-1714. [ Ref ]