Journal Name: Journal of Pediatrics and Infants
Article Type:Research Article
Received date:03 December, 2020
Accepted date:04 January, 2021
Published date:11 January, 2021
Citation: Agüera-Arenas JJ, Viedma MV, Martínez C, Micol O, Alcaraz M (2021) Less Invasive Surfactant Administration via Thin Catheter in Late Preterm Infants with Respiratory Distress Syndrome. J Pediat Infants Vol: 4, Issu: 1 (11-17).
Copyright: © 2021 Agüera-Arenas JJ et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: The use of less invasive surfactant administration (LISA) has been increasingly investigated in neonatal respiratory distress syndrome (RDS). However, this technique has been barely studied in late preterm newborns. We analyzed the use of LISA using a thin catheter in late preterm infants with RDS who required non-invasive ventilation (NIV).
Methods: A retrospective study was conducted on late preterm infants between June 2017 and March 2020. Poractant alfa was administered by LISA procedure. Maternal and prenatal data, as well as technique-related variables were collected. Statistical analyses were performed using SPSS version 20.0.
Results: A total of 20 patients were included, with a mean gestational age of 35 (341/7-366/7) weeks. Seven infants (35.0%) were exposed to prenatal corticosteroids. Mothers had a mean age of 31.0 (16-40) years. Half of newborns required resuscitation to breathe, with no signs of acute fetal distress. Premedications administered were caffeine (50.0%) and ketamine/morphine sedation (20.0%). Response to LISA was favorable, with a rapid and sustained reduction in FiO2 (FiO2 0.21 in 85.0% of patients at 24 h), and NIV mean time of 70 hours. Five patients (25%) did not experience any complications, and the rest mainly showed a small decrease in oxygen saturation. Redosing was needed in one of patients diagnosed with RDS and none of them required invasive mechanical ventilation.
Conclusion: LISA procedure for surfactant delivery in late premature infants with RDS was effective and safe, improving respiratory outcomes with a low incidence of complications and no need for IMV.
Keywords: Late preterm infants, Less Invasive Surfactant Administration (LISA), Poractant alfa, Respiratory Distress Syndrome (RDS), Surfactant therapy.
Abbreviations: BPD: Bronchopulmonary dysplasia, FiO2: Fraction of inspired oxygen, IMV: Invasive mechanical ventilation, IVH: Intraventricular hemorrhage, LISA: Less Invasive Surfactant Administration, MIST: Minimally Invasive Surfactant Therapy, nCPAP: Nasal continuous positive airway pressure, NEC: Necrotizing enterocolitis, NICU: Neonatal Intensive Care Unit, NIV: Non-invasive ventilation, nIPPV: Nasal intermittent positive pressure ventilation, PEEP: Positive end-expiratory pressure, RDS: Respiratory distress syndrome, SpO2: Peripheral capillary oxygen saturation.
Background
Respiratory distress syndrome (RDS), also known as hyaline membrane disease, is the most common respiratory disorder experienced by premature infants, as well as the leading cause of neonatal respiratory morbidity and mortality [1]. The condition is caused by lack of surfactant in the lungs, which leads to atelectasis, decreased gas exchange and hypoxia [2]. The incidence of RDS decreases with advancing gestational age, from about 60–80% in babies born at 26–28 weeks, to about 15–30% in those born at 32–36 weeks [3,4]. Among premature newborns, late preterm infants —those born between 340/7 and 366/7 weeks of gestation— account for approximately 70% of all preterm births [5,6]. Although these infants have been historically considered to be developmentally as mature as term newborns, they are known to be structurally and functionally immature, and have significantly higher rates of mortality and respiratory morbidities, such as RDS, than term infants [7-10]. Management of RDS is therefore primarily focused on providing appropriate respiratory support to maximize survival while reducing potential complications.
Surfactant therapy is a well-established therapy for RDS in preterm neonates [11,12] and plays an essential role in the management of RDS in late preterm infants [13,15]. Administration of surfactant can be performed by standard endotracheal intubation, requiring experienced practitioners, or through less invasive surfactant administration (LISA) techniques, also referred to as MIST (Minimally Invasive Surfactant Therapy). The most commonly utilized LISA technique involves the use of a thin catheter, which is being used as a non-invasive alternative to the standard mode of surfactant delivery [11,15-19]. This technique is becoming widely employed in neonatal intensive care units (NICU) worldwide [20-22], since it reduces the need for mechanical ventilation and decreases the risk of bronchopulmonary dysplasia (BPD) in preterm infants [15,23-26]. However, research has been mainly focused on very premature infants, and the potential benefit of surfactant administration using LISA procedure in late preterm neonates requires further investigation. In this study, we investigated the tolerability and the effects of the use of LISA through a thin catheter in late preterm infants with RDS admitted to our NICU.
Methods
Study design and participants
This was a retrospective, single-center, observational study conducted between June 2017 and March 2020, in a level III NICU at Virgen de la Arrixaca University Hospital in Murcia (Spain).
Patients that met the following criteria were included: preterm infants between 340/7 and 366/7 weeks of gestation with clinical and radiographic evidence of neonatal RDS that required non-invasive ventilation (NIV) as initial support, provided with either nasal continuous positive airway pressure (nCPAP) or nasal intermittent positive pressure ventilation (nIPPV); patients requiring a fraction of inspired oxygen (FiO2) ≥30% and with a positive end-expiratory pressure (PEEP) of 6 cm of H2O to maintain an oxygen saturation level (SpO2) ≥90%, who were administered exogenous pulmonary surfactant (poractant alfa) by direct laryngoscopy via thin catheter (vascular catheter or LISAcath®).
Procedures
LISA procedure involved the use of a laryngoscope to place the thin catheter beyond the vocal cords to the required depth. Once the catheter was correctly positioned, poractant alfa was dosed at 200 mg/kg for the first dose and then at 100 mg/kg every 6-8 hours, for up to 2 additional doses, while maintaining nCPAP or nIPPV. Surfactant administration was divided into slow boluses of 0.5 ml each administered through the catheter, according to the patient tolerability. During and after surfactant instillation, aspiration of gastric contents was performed to confirm whether surfactant reached the lungs effectively, and if an additional surfactant administration was required.
Premedication administered in newborns of less than 35 weeks’ gestation included atropine and caffeine, to reduce the frequency of apnea of prematurity. Various nonpharmacological methods were used as analgesic measures to alleviate pain in neonates, such as oral sucrose, non-nutritive sucking, and contention measures. Pharmacological methods included the administration of the anesthetics propofol (0.5 mg/kg), morphine, and ketamine (1 mg/kg), when needed.
Data recording
Maternal and neonatal data, collected retrospectively from the medical records, included sex, gestational age, birth weight, intrauterine growth retardation cases, multiple birth, type of delivery (eutocic, C-section or instrumental); Apgar score at 1, 5 and 10 minutes; need for cardiopulmonary resuscitation intervention with nCPAP or nIPPV; maternal age, prenatal corticosteroids, vertical sepsis, maternal gestational pathologies, first admission unit (intermediate neonatal care unit or NICU); type of NIV before LISA (nCPAP or nIPPV); FiO2 before and at 1, 6, 24, 56 and 72 h after surfactant treatment; pCO2 and pH values obtained from capillary blood gas measurements before LISA; chronological age at surfactant administration; number of surfactant doses administered; drug administration before or during LISA (atropine, caffeine, and/or sedative medications); desaturation events during LISA (mild: SpO2 80-89%; moderate: SpO2 60-80%; severe SpO2 <60%; NIV duration; need for invasive mechanical ventilation (IMV) after LISA; neonatal morbidities such as BPD, necrotizing enterocolitis (NEC) and intraventricular hemorrhage (IVH); final diagnosis; duration of NICU stay; and duration of neonatal hospital stay.
Statistical analysis
Descriptive statistics were generated for all data. Continuous variables were described using the median and range (described by the minimum and maximum values of the variables). Categorical variables were described as absolute (n) and relative (%) frequencies. All statistical analyses were performed using SPSS version 20.0.
Results
The number of patients included was 20, who represented the 33.3% of all late preterm neonates with respiratory failure that required NIV and who were admitted to our NICU during the study period. Demographic and clinical characteristics of mothers and preterm infants receiving surfactant with LISA technique are shown in table 1. The mean gestational age was 35 (341/7-366/7) weeks. Seven infants (35.0%) received prenatal corticosteroids for fetal lung maturation. Mothers had a mean age of 31.0 (16-40) years and only one of them presented an underlying medical condition (HELLP syndrome). The most frequent type of delivery was C-section (15/20, 75.0%), and the multiple birth rate was 35.0% (7/20). Only three preterm infants (15.0%) were hospitalized on the first day of admission at the NICU, whereas the rest of neonates (17/20, 85.0%) were admitted to an intermediate neonatal care unit. Ten newborns (50.0%) required resuscitation to breathe with nCPAP or nIPPV, although no signs of acute fetal distress were observed (all births had a 5 min Apgar score >5). Mean pCO2, pH and FiO2 values before LISA were 53 (40-81) mmHg, 7.28 (7.19-7.48), and 0.41 (0.3-0.6), respectively. Before LISA, thirteen patients (13/20, 65.0%) were treated with nCPAP and seven (7/20, 35.0%) with nIPPV. Premedications used before or during LISA were caffeine, which was administered to 50.0% (10/20) of infants; and ketamine or morphine sedation, in 20.0% (4/20) of cases.
Table 2 summarizes the characteristics of surfactant administration with LISA and the respiratory evolution of the preterm infants. The mean age at surfactant administration was 22 (2-48) hours of life. A LISAcath® catheter was used in 85% (17/20) of patients, whereas a vascular catheter was used in the rest of cases (15%, 3/20).
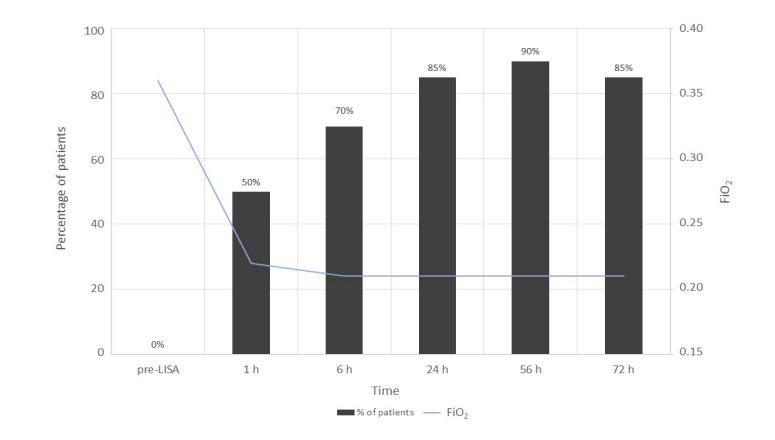
Figure 1 shows the evolution of FiO2 levels with time — before and up to 72 h after surfactant administration— and the percentage of patients that reached a FiO2 value of 0.21 at each time point. Median FiO2 prior to administration of surfactant was 0.36. One hour after the procedure, half of infants reached a FiO2 of 0.21, and 9 of them had a FiO2 ≤ 0.35 before LISA. The percentage of patients that reached a FiO2 of 0.21 increased to 70.0% at 6 h post-administration. The vast majority of patients (90.0%) reached FiO2 0.21 after 56 h, remaining at this value after 72 h of instillation except for one infant, whose FiO2 increased to 0.30 probably because NIV was discontinued.
The mean time on NIV was 70 (24-168) hours. Overall, the final diagnosis was RDS in 18 (90.0%) infants, one congenital pneumonia (5.0%) and one pulmonary interstitial glycogenosis (5.0%). None of the patients with a final diagnosis of RDS required IMV. In addition, no severe comorbidities as well as no deaths occurred. The nonRDS patients improved their condition after surfactant administration, with a decrease in FiO2 requirement, although improvement was transient and not sustained. The only infant requiring IMV was the one diagnosed with congenital pneumonia. The intrauterine growth-retarded infant was diagnosed with periventricular leukomalacia of prenatal origin in the first cerebral ultrasound at 3 days of life. The mean duration of NICU stay was 6 (2-23) days and the mean duration of hospital stay was 17 (7-39) days.
During LISA procedure, five patients (25%) did not experience any complications, whereas the rest (75.0%) had a decrease in oxygen saturation (SpO2) levels at some time during the intervention: 10 cases (50.0%) showed a mild decrease (SpO2 : 80-89%), 3 (15.0%) were moderate (SpO2 :60-80%), and 2 (10.0%) severe (SpO2 <60%). LISA was interrupted in those patients that underwent severe oxygen desaturation. One of them also experienced surfactant reflux, apnea, and coughing. However, none of them required intubation. Surfactant redosing was needed in one patient with RDS, who received surfactant by LISA, and another one with a final diagnosis of congenital pneumonia, who received endotracheal intubation. Infants whose mothers received prenatal corticosteroids either had a mild decrease in oxygen saturation or did not present any complications during the procedure.
| Characteristic | Infants analyzed (n=20) |
|---|---|
| Sex, n (%) | |
| Male | 12 (60.0) |
| Female | 8 (40.0) |
| Gestational age (weeks) | 35 (341/7-366/7) |
| Distribution of gestational ages, n (%) | |
| 341/7-346/7 | 11 (55.0) |
| 351/7-356/7 | 6 (30.0) |
| 361/7-366/7 | 3 (15.0) |
| Birth weight (g) | 2,363 (1,640-3,300) |
| Intrauterine growth retardation cases, n (%) | 1 (5.9) |
| Multiple birth, n (%) | 7 (35.0) |
| Type of delivery, n (%) | |
| Eutocic | 5 (15.0) |
| Cesarean section | 15 (75.0) |
| Instrumental | 0 (0.0) |
| Apgar score >5 at 5 minutes, n (%) | 20 (100.0) |
| Need for cardiopulmonary resuscitation intervention with nCPAP or nIPPV, n (%) | 10 (50.0) |
| nCPAP | 7 (35.0) |
| nIPPV | 3 (15.0) |
| Maternal age (years) | 31 (16-40) |
| Prenatal corticosteroids, n (%) | 7 (35.0) |
| Vertical sepsis, n (%) | 3 (15.0) |
| Maternal gestational pathologies, n (%) | 1 (5.0) |
| First admission unit | |
| Intermediate neonatal care unit, n (%) | 17 (85.0) |
| NICU, n (%) | 3 (15.0) |
| First admission unit | |
| nCPAP, n (%) | 13 (65.0) |
| nIPPV, n (%) | 7 (35.0) |
| Maximum FiO2 | 0.40 (0.3-0.6) |
| pH before LISA | 7.28 (7.19-7.48) |
| pCO2 before LISA (mmHg) | 53 (40-81) |
| Data are presented as mean (range) or n (%). FiO2 : fraction of inspired oxygen; LISA: Less Invasive Surfactant Administration; nCPAP: nasal continuous positive airway pressure; nIPPV: nasal intermittent positive pressure ventilation; NICU: Neonatal Intensive Care Unit; NIV: noninvasive ventilation. | |
Table 1: Demographic and clinical characteristics of mothers and infants receiving surfactant administration with LISA technique.
| Characteristic | Infants analyzed (n=20) |
|---|---|
| Chronological age at surfactant administration (hours) | 22 (2-48) |
| Number of surfactant doses administered, n (%) | |
| One dose | 18 (90.0) |
| Two doses | 2 (10.0) |
| Drug administration before or during LISA, n (%) | |
| Atropine | 20 (100.0) |
| Caffeine | 10 (50.0) |
| Sedative medications | 4 (20.0) |
| Desaturation events during LISA, n (%) | |
| None | 5 (25.0) |
| Mild (SpO2 80-89%) | 10 (50.0) |
| Moderate (SpO2 60-80%) | 3 (15.0) |
| Severe (SpO2 <60%) | 2 (10.0) |
| NIV duration (hours) | 70 (24-168) |
| Need for IMV after LISA, n (%) | 1 (5.9)1 |
| Neonatal morbidities (BPD, NEC, IVH), n (%) | 0 (0.0) |
| Final diagnosis, n (%) | |
| RDS | 18 (90.0) |
| Congenital pneumonia | 1 (5.0) |
| Pulmonary interstitial glycogenosis | 1 (5.0) |
| FiO2 of 0.21 at 72 hours of life, n (%) | 17 (85.0) |
| Duration of NICU stay (days) | 6 (2-23) |
| Duration of neonatal hospital stay (days) | 17 (7-39) |
| Death, n (%) | 0 (0.0) |
| Data are presented as mean (range) or n (%). 1: neonate diagnosed with pulmonary interstitial glycogenosis. BPD: bronchopulmonary dysplasia; FiO2: fraction of inspired oxygen; IMV: invasive mechanical ventilation; IVH: intraventricular hemorrhage; LISA: Less Invasive Surfactant Administration; NEC: necrotizing enterocolitis; NICU: Neonatal Intensive Care Unit; NIV: noninvasive ventilation; RDS: respiratory distress syndrome. | |
Table 2: Characteristics of surfactant administration with LISA and respiratory evolution of the late preterm infants.
Figure 1:Evolution of median FiO2 levels before and after surfactant administration, and percentage of patients that reached a FiO2 value of 0.21 at each time point.
Discussion
In this retrospective study, we assessed the effectiveness and tolerability of surfactant administration using the LISA technique in late preterm infants diagnosed with RDS.
Among all preterm newborns, half of them required resuscitation with nCPAP or nIPPV as initial respiratory support. Although these patients experienced difficulties during transition from intrauterine to extrauterine life, none of them had signs of acute fetal distress. Nonetheless, only three infants (15.0%) were hospitalized on the first day of admission at NICU, indicating a late RDS detection in the preterm newborns first admitted to an intermediate neonatal care unit. This fact emphasizes the importance of finding alternative methods for the diagnosis of RDS that are not based exclusively on clinical features and radiographic findings. On the other side, this would explain the initial higher FiO2 values and mean chronological age at surfactant administration of those preterm newborns first admitted to an intermediate neonatal care unit, as compared to the rest of preterm infants admitted to the NICU (mean FiO2 : 0.40 versus 0.37; mean age: 22 versus 8.5 h).
After surfactant administration using LISA, we found an important and sustained improvement of the respiratory function in the vast majority of patients, as evidenced by the decrease in FiO2 to 0.21 at 24 h in 85% of infants. Interestingly, 9 of 10 patients that immediately responded to surfactant administration reaching a FiO2 of 0.21 in the first hour after treatment had a FiO2 ≤0.35 before LISA. As a consequence, a cut-off value of initial FiO2 to predict a favorable response to LISA could be established at ≤0.35.
On the other side, patients with a final diagnosis of RDS did not require NIV after surfactant treatment. Despite the fact that two patients were clinically misdiagnosed with RDS, LISA procedure did not have any negative impact on them. On the contrary, FiO2 levels were reduced after LISA, and IMV was only required in one of the cases —the preterm newborn diagnosed with congenital pneumonia—. The infant diagnosed with pulmonary glycogenosis did not require IMV, except to perform lung biopsy, through which the definitive diagnosis was made. However, the observed clinical improvements were transient and clinicians suspected that patients could have been initially misdiagnosed with RDS. Again, these observations highlight the need for a correct diagnosis of RDS, not only based on radiological findings and clinical course. We believe that the use of thoracic ultrasonography, which is considered an accurate and reliable tool in the diagnosis of RDS in the newborn [27,28], could help early and rigorous diagnosis of the disease.
While the efficacy of surfactant therapy has been reported to improve respiratory function in late preterm infants with RDS [13,29,30], surfactant administration by minimally invasive methods is still being investigated in this population. The LISA method via thin catheter is widely adapted in Europe [31] and is a recommended method of care according to the latest consensus guidelines [11,15,32]. However, there is still large variability in the application of this technique regarding patient population that may benefit from this approach; the catheter type, the adequate dose and type of surfactant; the use of premedication; and the need of pharmacologic sedation during the procedure [33]. A meta-analysis conducted by Lau et al. [18] provides a comprehensive review of the use of LISA via thin catheter in preterm infants. Overall, the authors underline —as reported in previous works [17,24,34,35]— the suitability of this technique in preterm infants. However, additional studies are required to address the infant selection issue in this vulnerable population. While the mentioned studies recommend the use of LISA in preterm infants, their outcomes should not be extrapolated to a different pediatric age range or other specific age subgroups. Up to date, there is only one study reporting on the use of LISA in moderate and late preterm neonates between 320/7 and 366/7 weeks [36]. The authors compared standard RDS management (surfactant administration given only after intubation) with LISA procedure. Their results showed a reduction in mechanical ventilation exposure and RDS complications as compared to standard therapy. In our study, the LISA procedure performed on late preterm infants appeared to be of benefit for this particular subgroup of premature babies, with no need for IMV after LISA, thus reducing its associated complications. Furthermore, the adverse events documented during LISA were low, with mainly mild to moderate desaturations and a small rate of surfactant reflux, indicating that surfactant delivery was optimal for the vast majority of patients [37].
The positive respiratory outcomes of surfactant treatment with LISA technique and the low frequency of acute side effects observed in this study could respond to various factors, such as the type of the catheter used; the type, dose, and volume of the surfactant administered; the use of premedication; and the extensive experience of our neonatologists performing LISA procedure. The type of catheters utilized for surfactant delivery were LISAcath® or semirigid vascular catheters, which are easier to handle, do not require Magill forceps, and lower the risk of injuries in premature infants. In relation to the effect of the surfactant, it has been reported that the size of the first dose may be more important for clinical response than the source of surfactant [38]. In our study, the initial dose of poractant alfa was 200 mg/kg, which has been shown to lead to a lower risk for complications than an initial dose of 100 mg/kg [38,39]. Higher first doses of surfactant also reduce the need for repeat doses [40,41], as shown in a study conducted in preterm infants <35 weeks gestation with RDS that required fewer additional doses of poractant alfa (200 mg/kg) as compared to beractant (100 mg/kg) [42]. Our findings are in accordance with these observations, since surfactant redosing was only required in one of the late preterm infants diagnosed with RDS. Exposure to antenatal corticosteroids also seemed to be associated with a low risk of procedural complications, as observed by either the absence of problems or a mild decrease in oxygen saturation during LISA. In addition, all but one of these patients reached a FiO2 of 0.21 after surfactant treatment. In fact, as it has been reported, administration of steroids in mothers at risk of late preterm delivery has been attempted with promising results in terms of a decreased need for respiratory support and oxygen [37].
Regarding the use of medications during the procedure, sedation and analgesia were administered at the discretion of each neonatologist, since there are not established protocols in our unit. In fact, administration of sedation and analgesia are controversial issues in RDS management [43]. More specifically, sedation for LISA is complex, because while low-dose sedation prior to laryngoscopy is technically attainable and makes the baby less uncomfortable, it increases the risk of CPAP failure. Thus, there is no clear consensus on whether or not to sedate routinely for LISA, and which sedative to use, leaving these decisions up to clinicians [11]. Our neonatologists have the subjective perception that in the absence of pharmacological sedation, technical complications during LISA arise more frequently in late preterm infants than in other premature newborns.
Our conclusions should be considered in light of some intrinsic limitations of the retrospective nature of this study. Secondly, the low sample size and the lack of a control group did not allow us to establish definitive conclusions on the effectiveness and tolerability of the technique, nor to compare outcomes with other modes of surfactant administration. Therefore, the results presented herein should be considered exploratory and interpreted with caution. Future studies with larger sample sizes will be needed to evaluate the benefits of LISA in late preterm infants, as well as to address unresolved questions and clinical issues for its successful implementation.
Conclusion
The results of this study may have relevance to clinical practice on the effect of the LISA technique via thin catheter in late preterm infants diagnosed with RDS. LISA appeared to be an effective and safe method of surfactant delivery in late premature neonates, improving respiratory outcomes with no need for mechanical ventilation. Moreover, adverse outcomes associated with LISA were low, with mainly mild oxygen desaturations, and a small rate of surfactant reflux and apnea.
Acknowledgement
Medical writing assistance was provided by Blanca Martínez-Garriga on behalf of Trialance (www.trialance.com).
Disclosure Statements
The authors of this manuscript received financial support from Chiesi España S.A.U. for the writing of the manuscript. The sponsor had no role in the design or conduct of the study, data collection and analysis, or preparation of the manuscript.
The authors declare that they have no competing interests.
Ersch J, Roth-Kleiner M, Baeckert P, Bucher HU (2007) Increasing incidence of respiratory distress in neonates. Acta Paediatr 96: 1577-1581.[ Ref ]
Thygesen SK, Olsen M, Østergaard JR, Sørensen HT (2016) Respiratory distress syndrome in moderately late and late preterm infants and risk of cerebral palsy: a population-based cohort study. BMJ Open 6: e011643. [ Ref ]
Gallacher DJ, Hart K, Kotecha S (2016) Common respiratory conditions of the newborn. Breathe (Sheff) 12: 30-42.[ Ref ]
Ventolini G, Neiger R, Mathews L, Adragna N, Belcastro M (2008) Incidence of respiratory disorders in neonates born between 34 and 36 weeks of gestation following exposure to antenatal corticosteroids between 24 and 34 weeks of gestation. Am J Perinatol 25: 79-83. [ Ref ]
Raju TN, Higgins RD, Stark AR, Leveno KJ (2006) Optimizing care and outcome for late-preterm (near-term) infants: a summary of the workshop sponsored by the National Institute of Child Health and Human Development. Pediatrics 118: 1207-1214.[ Ref ]
Gouyon JB, Vintejoux A, Sagot P, Burguet A, Quantin C, et al. (2010) Neonatal outcome associated with singleton birth at 34-41 weeks of gestation. Int J Epidemiol 39: 769-776. [ Ref ]
Leone A, Ersfeld P, Adams M, Meyer PS, Bucher HU, et al. (2012) Neonatal morbidity in singleton late preterm infants compared with full-term infants. Acta Paediatr 101: 6-10.[ Ref ]
Shapiro-Mendoza CK, Tomashek KM, Kotelchuck M, Barfield W, Nannini A, et al. (2008) Effect of late-preterm birth and maternal medical conditions on newborn morbidity risk. Pediatrics. 121: e223-232. [ Ref ]
McIntire DD, Leveno KJ (2008) Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol 111:35-41. [ Ref ]
Williams JE, Pugh Y (2018) The Late Preterm: A Population at Risk. Crit Care Nurs Clin North Am 30: 431-443.[ Ref ]
Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, et al. (2019) European Consensus Guidelines on the Management of Respiratory Distress Syndrome - 2019 Update. Neonatology 115: 432-450. [ Ref ]
Jobe AH (1993) Pulmonary surfactant therapy. N Engl J Med 328: 861-868.[ Ref ]
Dani C, Mosca F, Vento G, Tagliabue P, Picone S, et al. (2018) Effects of surfactant treatment in late preterm infants with respiratory distress syndrome. J Matern Fetal Neonatal Med 31: 1259-1266. [ Ref ]
Sürmeli-Onay O, Korkmaz A, Yiğit S, Yurdakök M (2012) Surfactant therapy in late preterm infants: respiratory distress syndrome and beyond. Turk J Pediatr 54: 239-246.[ Ref ]
Banerjee S, Fernandez R, Fox GF, Goss KCW, Mactier H, et al. (2019) Surfactant replacement therapy for respiratory distress syndrome in preterm infants: United Kingdom national consensus. Pediatr Res 86: 12-14. [ Ref ]
Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, et al. (2017) European Consensus Guidelines on the Management of Respiratory Distress Syndrome - 2016 Update. Neonatology 111: 107-125. [ Ref ]
Aguar M, Cernada M, Brugada M, Gimeno A, Gutierrez A, et al. (2014) Minimally invasive surfactant therapy with a gastric tube is as effective as the intubation, surfactant, and extubation technique in preterm babies. Acta Paediatr 103: e229-233. [ Ref ]
Lau CSM, Chamberlain RS, Sun S (2017) Less Invasive Surfactant Administration Reduces the Need for Mechanical Ventilation in Preterm Infants: A Meta-Analysis. Glob Pediatr Health. 4: 2333794X17696683.[ Ref ]
Herting E, Härtel C, Göpel W (2019) Less invasive surfactant administration (LISA): chances and limitations. Arch Dis Child Fetal Neonatal Ed 104: F655-F659. [ Ref ]
Isayama T, Chai-Adisaksopha C, McDonald SD (2015) Noninvasive ventilation with vs without early surfactant to prevent chronic lung disease in preterm infants: a systematic review and meta-analysis. JAMA Pediatr 169: 731-739.[ Ref ]
More K, Sakhuja P, Shah PS (2014) Minimally invasive surfactant administration in preterm infants: a meta-narrative review. JAMA Pediatr 168: 901-908.[ Ref ]
Vento M, Bohlin K, Herting E, Roehr CC, Dargaville PA (2019) Surfactant Administration via Thin Catheter: A Practical Guide. Neonatology 116: 211-226.[ Ref ]
Aldana-Aguirre JC, Pinto M, Featherstone RM, Kumar M (2017) Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 102: F17-F23.[ Ref ]
Kribs A, Roll C, Göpel W, Wieg C, Groneck P, et al. (2015) NINSAPP Trial Investigators. Nonintubated Surfactant Application vs Conventional Therapy in Extremely Preterm Infants: A Randomized Clinical Trial. JAMA Pediatr 169: 723-730.[ Ref ]
Göpel W, Kribs A, Ziegler A, Laux R, Hoehn T, et al. (2011) German Neonatal Network. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an openlabel, randomised, controlled trial. Lancet 378: 1627-1634.[ Ref ]
Isayama T, Iwami H, McDonald S, Beyene J (2016) Association of Noninvasive Ventilation Strategies With Mortality and Bronchopulmonary Dysplasia Among Preterm Infants: A Systematic Review and Meta-analysis. JAMA 316: 611-624.[ Ref ]
Oktem A, Yigit S, Oğuz B, Celik T, Haliloğlu M, et al. (2019) Accuracy of lung ultrasonography in the diagnosis of respiratory distress syndrome in newborns. J Matern Fetal Neonatal Med 22: 1-6. [ Ref ]
Liu J, Cao HY, Wang HW, Kong XY (2014) The role of lung ultrasound in diagnosis of respiratory distress syndrome in newborn infants. Iran J Pediatr 24: 147-154.[ Ref ]
Wirbelauer J, Speer CP (2009) The role of surfactant treatment in preterm infants and term newborns with acute respiratory distress syndrome. J Perinatol 29 Suppl 2: S18-S22.[ Ref ]
Collaborative Study Group for Late-preterm/Term Infants with Respiratory Distress (2014) A multicenter study on the surfactant treatment in late-preterm or term infants with respiratory distress syndrome. Multicenter Study 52: 724-728.[ Ref ]
Klotz D, Porcaro U, Fleck T, Fuchs H (2017) European perspective on less invasive surfactant administration-a survey. Eur J Pediatr 176: 147-154. [ Ref ]
Shim GH (2017) Update of minimally invasive surfactant therapy. Korean J Pediatr 60: 273-281. [ Ref ]
Niemarkt HJ, Hütten MC, Kramer BW (2017) Surfactant for Respiratory Distress Syndrome: New Ideas on a Familiar Drug with Innovative Applications. Neonatology 111: 408-414. [ Ref ]
Dargaville PA, Aiyappan A, De Paoli AG, Kuschel CA, Kamlin CO, et al. (2013) Minimally-invasive surfactant therapy in preterm infants on continuous positive airway pressure. Arch Dis Child Fetal Neonatal Ed. 98: F122-F126.[ Ref ]
Krajewski P, Chudzik A, Strzałko-Głoskowska B, Górska M, Kmiecik M, et al. (2015) Surfactant administration without intubation in preterm infants with respiratory distress syndrome--our experiences. J Matern Fetal Neonatal Med 28: 1161-1164. [ Ref ]
Olivier F, Nadeau S, Bélanger S, Julien AS, Massé E, et al. (2017) Efficacy of minimally invasive surfactant therapy in moderate and late preterm infants: A multicentre randomized control trial. Paediatr Child Health 22: 120-124. [ Ref ]
García-Reymundo M, Demestre X, Calvo MJ, Ginovart G, Jiménez A, et al. (2018) Prematuro tardío en España: experiencia del Grupo SEN 34-36. Anales de Pediatría 88: 246-252. [ Ref ]
Singh N, Halliday HL, Stevens TP, Suresh G, Soll R, et al. (2015) Comparison of animal-derived surfactants for the prevention and treatment of respiratory distress syndrome in preterm infants. Cochrane Database Syst Rev 21: CD010249.[ Ref ]
Tridente A, De Martino L, De Luca D (2019) Porcine vs bovine surfactant therapy for preterm neonates with RDS: systematic review with biological plausibility and pragmatic meta-analysis of respiratory outcomes. Respir Res 20: 28. [ Ref ]
Bohlin K, Gudmundsdottir T, Katz-Salamon M, Jonsson B, Blennow M (2007) Implementation of surfactant treatment during continuous positive airway pressure. J Perinatol 27: 422-427.[ Ref ]
Hentschel R, Bohlin K, van Kaam A, Fuchs H, Danhaive O (2020) Surfactant replacement therapy: from biological basis to current clinical practice. Pediatr Res 88: 176-183.[ Ref ]
Ramanathan R, Rasmussen MR, Gerstmann DR, Finer N, Sekar K, et al. (2004) A randomized, multicenter masked comparison trial of poractant alfa (Curosurf) versus beractant (Survanta) in the treatment of respiratory distress syndrome in preterm infants. Am J Perinatol 21: 109-119. [ Ref ]
McPherson C, Inder T (2017) Perinatal and neonatal use of sedation and analgesia. Semin Fetal Neonatal Med 22: 314-320.[ Ref ]