Journal Name: Journal of Pediatrics and Infants
Article Type: Retrospective Study
Received date: 04 December, 2020
Accepted date:26 January, 2021
Published date:02 February, 2021
Citation:Fainardi V, Bonacini I, Sapienza E, Corradi M, Magnani C et al. (2021) Lung Function in a Cohort of Italian Children Born Preterm. J Pediat Infants Vol: 4, Issu: 1 (32-39).
Copyright: © 2021 Fainardi V et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background and aim: in children born preterm lung development may be impaired with diminished lung function in later ages. The aim of the study was to investigate lung function in a cohort of school-aged children born preterm assessing the influence of perinatal variables.
Methods: we measured lung function with impulse oscillometry system (IOS) and spirometry in 54 children [(male 48.1%, mean age 8.1 (0.8)] born preterm [mean birth weight (BW) 1462 (546.9) g, mean gestational age 31.5 (3) weeks] at Parma Children University Hospital (Italy). Maternal and perinatal data and respiratory medical history were also collected.
Results: compared to predicted values, children born preterm showed higher mean values of airway impedance Z, airway resistance R5, R20, and R5-20, area under the reactance curve AX and resonance frequency Fres, lower mean values of reactance X5 and FEF25-75 were recorded. The mean difference between observed and predicted values of R5-R20 was higher in children born small for gestational age than in those born appropriate for gestational age. An inverse relationship was found between BW z-score and Z (r -0.40, r² 0.16; p 0.012) and R5 (r -0.44, r² 0.20; p 0.005) in children born with a BW <1500 g. Thirty-one per cent of children had a history of wheezing and 14.8% a history of lower respiratory infection requiring admission to the hospital.
Conclusion: school-aged children born preterm had an impaired lung function, especially in the peripheral airways, as resulted by IOS and spirometry assessment. BW may have a role in lung development.
Key words: Impulse Oscillometry System, IOS, Prematurity, Small for Gestational Age, IUGR
List of Abbreviations: IOS impulse oscillometry system, BW birth weight, GA gestational age, Z impedance, X5 reactance at 5 Hz, R5 resistance at 5 Hz, R20 resistance at 20 Hz, AX area under the reactance curve, Fres resonance frequency, VLBW very low birth weight , BPD bronchopulmonary dysplasia, SGA small for gestational age , AGA appropriate for gestational age, SD standard deviation, GLI global lung initiative, ISAAC International Study of Asthma and Allergy in Childhood, FOT forced oscillation technique, IUGR intrauterine growth restriction.
Introduction
Preterm birth rate ranges from 5% to 10% of live births depending on the geographical area and a very low birth weight (VLBW) <1500 g is reported in 1% of all infants [1,2]. Over the years, infant survival is improved but respiratory morbidity in the short and in the long-term is still an important complication.
Depending on gestational age (GA), fetal lung development is interrupted by preterm birth during the canalicular, saccular or alveolar phase of lung maturation. Perinatal exposures to inflammation, infection, mechanical ventilation and hyperoxia may lead to further insult to the immature lung with potentially long-term consequences on lung function. Bronchopulmonary dysplasia (BPD) is the most significant respiratory disease related to prematurity but the spectrum of respiratory impairment is wide involving also children without exposure to oxygen therapy or children born late preterm. Although the growth of the lung may catch-up during the first years of life [3], followup studies of subjects born preterm showed a higher risk of wheezing [4] and airway obstruction regardless a diagnosis of BPD [5,6]. These results suggest that preterm birth alone can negatively affect lung development [7,8] with potential consequences persisting into adulthood [9,10].
The aim of the present study was to investigate lung function using the impulse oscillometry system (IOS) and spirometry in school-aged children born preterm who were followed-up in the outpatient clinic of Parma Children University Hospital (Italy) assessing the associations between lung function and perinatal variables.
The study
Study population and data collection
Lung function data of children born preterm (GA <37 weeks) at Parma University Hospital (Italy) in the two-year periods 2005-2006 and 2009-2010 were collected. Children were assessed in the outpatient clinic of the Pediatric Respiratory Unit during the standard follow-up of children born preterm. Subjects with concomitant chronic cardiac disease or neuromuscolar disease were excluded from the study. Maternal (age, smoking during pregnancy, pregnancy complications, gestational diabetes, prenatal steroid administration, cause and type of delivery) and neonatal (sex, weight, length and cranial circumference at birth, Apgar score, surfactant administration, duration of invasive and non-invasive ventilation, duration of oxygen therapy and BPD diagnosis) data were collected from medical records.
A diagnosis of BPD was defined as oxygen need for >28 days from birth until 36 weeks of postmenstrual age according to the definition described elsewhere [11].
The study was approved by the ethical committee of Parma University Hospital (protocol number 732/2018/ OSS*7AOUPR).
Lung function tests
IOS and spirometry were performed using the Spirometry-IOS system (CareFusion, Germany). The system was calibrated each day prior to the measurements using a 3-liter syringe.
To obtain a valid IOS maneuver the subject had to tidally breath into a mouthpiece for at least 30 seconds with the cheeks supported by the hands of parents or operator. At least 3 reproducible maneuvers without artifacts due to coughing, swallowing, vocalization or breath holding had to be obtained to consider valid the tests. The mean value of IOS indices [impedance at 5 Hz (Z5), reactance at 5 Hz (X5), resistance at 5 Hz (R5) and at 20 Hz (R20), area under the reactance curve (AX) and resonance frequency (Fres)] were calculated from the 3 maneuvers. IOS indices (X5, Z5, R5, R20, AX and Fres) were expressed as raw values and z-scores derived from published reference data from a Brasilian healthy population; a log base-10 transformation of the variables Z5, R5, R20, Fres and AX in females and of the variable AX for males was performed according to the reference equations [12]. The difference R5-R20 was calculated and the predicted value derived on the basis of the predicted values of R5 and R20.
IOS generates small pressure oscillations at 30s intervals into the airways by a loud speaker and measures respiratory impedance Z that includes respiratory resistance (R5 and R20) and respiratory reactance X. The pulmonary resistance R is the in-phase component of the lung impedance Z and accounts for the energy required to propagate the pressure wave through the airways including the pulmonary parenchyma and the thorax. Because oscillation frequencies <15 Hz can be transmitted more distally in the lungs compared to higher frequencies, R5 reflects obstruction in both small and large airways while R20 reflects large airways only. The difference between R5 and R20 (R5-R20) is a derived index that describes the small airways resistance [13]. Reactance X is the out-of-phase component related to the elastic recoil and inertial properties of the lung. AX is the total reactance (area under the reactance curve) at all frequencies between 5 Hz and Fres (the point where the total reactance is 0) and reflects the ability of the peripheral lung to store capacitative energy (i.e. compliance). As the peripheral lung becomes less compliant, it cannot store as much capacitative energy and requires a larger pressure to inflate: an increase in small airway wall tone will decrease the reactance and increase AX. Thus, X5, AX and Fres all reflect changes in the degree of obstruction in the peripheral airways13. IOS was performed prior to spirometry to avoid influence of forced exhalation maneuvers on results.
Standard spirometry maneuvers were performed according to ATS/ERS standards [14]. Spirometric values (FEV1 , FVC, FEF25-75) were expressed as z-scores according to the Global Lung Initiative (GLI) reference values [15].
At the time of assessment children were clinically stable and none had shown respiratory infections in the previous 3 weeks. The respiratory medical history was recorded by means of the International Study of Asthma and Allergy in Childhood (ISAAC) questionnaire [16].
Data analysis
Data are reported as number and proportions or mean and standard deviations (SD) or median and 10th and 90th centile when appropriate. Descriptive statistics was used for differences in proportions. Associations between maternal or perinatal variables (preclampsia or eclampsia, gender, GA, BW z-score, surfactant administration, days of invasive ventilation and days of oxygen therapy) and lung function tests (Z, X5, R5, R20, R5-R20, AX, Fres, FEV1 , FVC, FEF25-75) were assessed by linear regression analysis and comparison between groups with Student’s t-test, as appropriate. Stepwise multiple regression analysis was used to determine the best predictor variables (sex, GA, BW z-score and preclampsia) for IOS parameters (Z, X5, R5, R20, R5-R20, AX, Fres) as dependent variables. Percentage of total variance in the dependent variable is expressed as the adjusted square of the multiple correlation coefficient (r²).
BW was converted to z-score according to the Italian specific reference for fetal growth [17]. Children were then categorized as born with a BW small for gestational age (SGA) when BW was ≥2SDs below the mean (i.e. <10th centile) or appropriate for gestational age (AGA) (i.e. ≥10th centile).
A lung function below the lower limit of normal was defined as a z-score less than -1.64 (<5th centile). The analyses were carried out with the SPSS Statistics software. A p value less than 0.05 was considered as significant.
Results
Population
In 2014 and 2019 fifty-five children [mean age 8.1 (0.87) years] in respiratory follow-up for prematurity at the outpatient clinic of the Paediatric Respiratory Unit of Parma Children University Hospital (Parma, Italy) were recruited. One girl was not included in the study because of severe neurological impairment. All children but one (98.1%) performed IOS test while only 44 (83%) were able to perform spirometry. The perinatal characteristics of these 54 children are shown in table 1.
Lung function
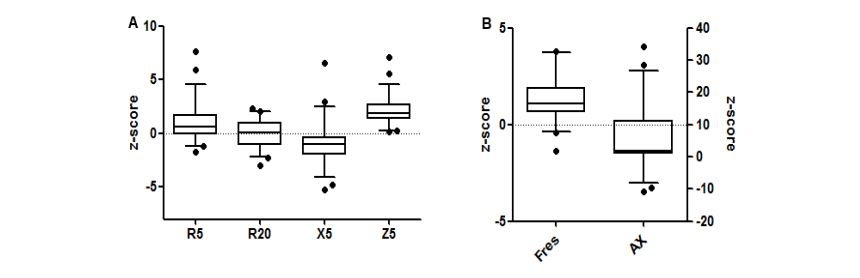
IOS measurements showed significantly higher impedance Z5, higher airway resistance (R5, R20, R5-R20), lower reactance X5, higher Fres and larger area under the reactance curve AX in children born preterm compared to predicted values (p <0.05). Box plots of distribution of IOS z-scores are reported in figure 1. Twenty-four per cent (n 13) of children had z-scores values above 95th centile for R5 and Fres and 11.1% (n 6) for R20; 27.7% (n 15) had values below the 5th centile for X5. Most children showed values above 95th centile for Z (57.4%, n 31) and AX (53.7%, n 29).
Compared to predicted values, children born preterm had a lower mean value of FEF25-75 (-0.31 L/sec, 95%CI -0.47;- 0.16, p <0.001) and males a lower FEV1 /FVC (-0.04, 95%CI -0.08;0.00 p 0.019). Five (11.3%) children showed values of FEV1 , FVC and FEF25-75 below the lower limit of normal (5th centile); 3 of them had a diagnosis of BPD. Patients with a diagnosis of BPD (n 10) showed a FEV1 z-score significantly lower than those without BPD [-0.99 (0.53) vs -0.05 (0.18), p 0.04]. A slight but not significant impairment was recorded in IOS parameters [Z 0.96 (0.08) vs 0.85 (0.03), p 0.178; R5 1.10 (0.81) vs 0.84 (0.21), p 0.662; X5 -1.54 (0.40) vs -0.83 (0.30), p 0.286; AX 8.73 (3.63) vs 5.55 (1.38), p 0.347; Fres 1.92 (0.61) vs 1.22 (0.15), p 0.117].
Mean raw and z-score values for IOS lung function and spirometry are shown in table 2.
A log base-10 transformation of the IOS variables Z5, R5, R20, Fres and AX in females and of the variable AX for males was performed according to the reference equations [12].
Gestational age, birth weight and lung function
Lung function (IOS and spirometry) did not change between children born preterm at < or > 32 weeks of gestation (data not shown). Fourteen children (25.9%) had a BW SGA. Spirometry indices did not differ between children born AGA and children born SGA. However, the mean difference between observed and predicted values of R5-R20 was higher in children born SGA than in those born AGA [(+0.21 (0.03) vs +0.13 (0.02), p 0.040]. No differences were found in the other IOS parameters. BW z-score showed a slight but not significant negative relationship with airway resistance [R5: r -0,23 (-0.74;0.06), p 0.094; R20: -0.09 (-0.45;0.21), p 0.490], impedance [Z: r -0.19 (-0.54;0.09), p 0.165] and area under the curve [AX -0.21 (-4.19;0.48), p 0.117]. In children born with a BW <1500 g, BW z-score was significantly inversely related to R5 and Z5. Relationships between IOS values and BW z-scores and GA as perinatal variables are reported in table 3. The regression equation generated by stepwise multiple regression analysis for Z5, R5, R20 and AX changes, as dependent variables, in children with BW <1500 g included male sex and BW z-score as independent variable (Table 4). In this model BW z-score accounted for 29%, 20% and 43% of the total variance for Z5, R5 and AX respectively. Male sex accounted for 22%, 28% and 34% for R20, Z5 and AX respectively.
| Characteristics | No of subjects (% or median) |
|---|---|
| Male | 26 (48.1%) | Gestational age, weeks |
| <28 | 5 (9.2%) |
| 28-<32 | 21 (38.9%) |
| 32-<37 | 28 (51.9%) |
| Median (10-90th centile) | 32 (26-35.2) |
| Birth weight, g | |
| <1500 | 37 (68.5%) |
| ≥1500 | 16 (31.5%) |
| Median (10-90th centile) | 1357.5 (810-2300) |
| Prenatal maternal steroids | 36 (66.6%) |
| Surfactant administration | 16 (29.6%) |
| Invasive ventilation | 17 (31.5%) |
| Non-invasive ventilation | 24 (44.4%) |
| Days on mechanical ventilation | |
| Median (10-90th centile) | 1 (0-3) |
| Oxygen therapy | 38 (70.3%) |
| Days on oxygen | |
| Median (10-90th centile) | 3 (0-47) |
| RDS | 24 (44.4%) |
| BPD | 10 (18.5%) |
| M: males; SGA: small for gestational age (Bertino E, 2010); RDS: respiratory distress syndrome; BPD: bronchopulmonary dysplasia, defined as supplemental oxygen dependency at 36 weeks of postmenstrual age. | |
Table 1: Characteristics of the cohort of 54 preterm babies.
Figure 1:Box plots (5-95°centile) showing the z-score distribution of R5, R20, X5, Z5 (A) and AX and Fres (B).
| Boys | Girls | ||||||
|---|---|---|---|---|---|---|---|
| Measured values | Predicted values | p | Measured values | Predicted values | p | ||
| R5 (kPa/L/s) | 0.76 (0.24) | 0.64 (0.08) | 0.021 | R5 (kPa/L/s) Log R5 (kPa/L/s) | 0.88 (0.19) -0.06 (0.09) | -0.11 (0.04) | 0.014 |
| z-score | 1.21 (2.16) | z-score | 0.58 (0.89) | ||||
| R20 (kPa/L/s) | 0.50 (0.12) | 0.56 (0.05) | 0.014 | R20 (kPa/L/s) Log R20 (kPa/L/s) | 0.62 (0.12) -0.20 (0.08) | -0.24 (0.03) | 0.023 |
| z-score | 0.77 (1.28) | z-score | 0.58 (1.03) | ||||
| R5-R20 (kPa/L/s) | 0.26 (0.17) | 0.07 (0.03) | <0.001 | R5-R20 (kPa/L/s) | 0.26 (0.11) | 0.13 (0.02) | <0.001 |
| X5 (kPa/L/s) | -0.24 (0.10) | -0.15 (0.03) | <0.001 | X5 (kPa/L/s) | -0.25 (0.11) | -0.21 (0.02) | 0.040 |
| z-score | -0.82 (0.90) | z-score | -1.10 (2.51) | ||||
| AX (kPa/L) Log AX (kPa/L) | 2.67 (1.75) 0.33 (0.28) | 0.06 (0.02) | <0.001 | AX (kPa/L) Log AX (kPa/L) | 2.86 (1.44) 0.40 (0.23) | 0.05 (0.08) | <0.001 |
| z-score | 11.1 (11.7) | z-score | 1.31 (0.79) | ||||
| Z5 (kPa/L/s) | 0.81 (0.24) | 0.48 (010) | <0.001 | Z5 (kPa/L/s) Log Z5 (kPa/L/s) | 0.93 (0.20) -0.03 (0.09) | -0.19 (0.05) | <0.001 |
| z-score | 2.40 (1.57) | z-score | 1.83 (0.89) | ||||
| Fres (Hz) | 23 (4.54) | 18.8 (2.02) | <0.001 | Fres (Hz) Log Fres (Hz) | 24.94 (3.48) 1.41 (0.12) | 1.26 (0.02) | <0.001 |
| z-score | 1.30 (1.40) | z-score | 1.40 (1.14) | ||||
| FEV1 (L) | 1.71 (0.41) | 1.60 (0.35) | 0.631 | FEV1 (L) | 1.42 (0.27) | 1.50 (0.18) | 0.254 |
| z-score | -0.25 (1.33) | z-score | -0.4 (1.25) | ||||
| FVC (L) | 2.05 (0.46) | 2.01 (0.38) | 0.776 | FVC (L) | 1.58 (0.34) | 1.68 (0.22) | 0.276 |
| z-score | 0.10 (1.15) | z-score | -0.45 (1.41) | ||||
| FEV1/FVC | 0.83 (0.09) | 0.88 (0.01) | 0.019 | FEV1/FVC | 0.90 (0.06) | 0.90 (0.0) | 0.823 |
| z-score | -0.51 (1.26) | z-score | 0.18 (1.10) | ||||
| FEF25-75 (L/sec) | 1.70 (0.54) | 2.07 (0.30) | 0.005 | FEF25-75 (L/sec) | 1.73 (0.34) | 1.99 (0.20) | 0.005 |
| z-score | -0.88 (1.18) | z-score | -0.50 (0.74) | ||||
| R5: resistance at 5 Hz; R20: resistance at 20 Hz; X5: reactance at 5 Hz; AX: area under the reactance curve; Fres: resonance frequency; Z5: impedance at 5 Hz; FEV1 :forced expiratory volume in the first second; FVC: forced vital capacity; FEF25-75: forced expiratory flow at 25-75% of the pulmonary volume. | |||||||
Table 2: IOS and spirometry values in the 54 preterm children. Predicted values for IOS and spirometry have been derived from published reference values [12,15].
| Z5 | X5 | R5 | R20 | AX | Fres | |
|---|---|---|---|---|---|---|
| BW z-score | -0.19 (-0.54;-0.09) | 0.02 (-0.42;0.52) | -0.23 (-0.74;-0.06) | -0.09 (-0.45-0.21) | -0.21 (-4.19;0.48) | -0.06 (-0.39;0.23) |
| BW <1500 g z-score | -0.40 (-0.85;-0.11)a | -0.01 (-0.53;0.49) | -0.44 (-1.13;-0.21)b | 0.25 (-0.79;0.10) | -0.25 (-5.62;0.75) | -0.21 (-1.46;0.48) |
| GA | 0.13 (-0.06;0.17) | 0.11 (-0.10;0.25) | 0.19 (-0.04;0.26) | 0.05 (-0.09;0.14) | -0.02 (-0.95;0.80) | -0.04 (-0.13;-0.10) |
| BW: birth weight; GA: gestational age. Z5: impedance at 5 Hz; X5: reactance at 5 Hz; R5: resistance at 5 Hz; R20: resistance at 20 Hz; AX: area under the reactance curve; Fres: resonance frequency. ap <0.05 bp <0.01 | ||||||
Table 3: Univariable linear regression models using lung function indices z-scores as the dependent variable and perinatal factors as independent factors. Data are presented as coefficients (95%CI).
| Z5 | R5 | R20 | AX | |
|---|---|---|---|---|
| BW z-score | -0.54 (-0.91;-0.16)b | -0.72 (-1.21;-0.22)b | ns | -3.0 (-5.71;-0.28)a |
| Male sex | 0.75 (0.51;1.45)a | ns | -1.22 (-2.02;-0.42)a | 11.1 (6.06;16.16)b |
| r² | 0.29 | 0.20 | 0.22 | 0.43 |
| For every model the following were tested as predictors: sex, gestational age, birth weight z-score and preeclampsia. BW: birth weight; GA: gestational age; ns: not significant. ap <0.05 bp <0.01 | ||||
Table 4: Stepwise multivariable linear regression models using lung function indices z-scores as the dependent variable and perinatal factors as independent factors in the cohort of children born <1500 g. Data are presented as coefficients (95%CI).
Reported respiratory symptoms
Thirty-one per cent of children (n 17) had a history of wheezing and 14.8% (n 8) a history of lower respiratory infection requiring admission to the hospital. Eleven children (20.3%) had experienced at least one episode of wheezing in the previous 12 months; there were no significant differences in lung function between these children and those without wheezing. Eight children (14.8%) were on regular antiasthmatic inhalers; 6 children were atopic (11.1%).
Discussion
In our cohort of school-aged children born preterm lung function measured by IOS and spirometry was significantly impaired as compared to predicted values. Notably, we found an increase in airway impedance with higher values of resistance and lower values of reactance, larger area under the reactance curve AX and higher resonant frequency Fres. Spirometry revealed normal forced expiratory volume and forced vital capacity but reduced values of FEF25-75 compared to predicted values. Children with a diagnosis of BPD showed a significant lower FEV1 than children without BPD. Having BPD did not influence IOS parameters while being born SGA was associated to higher increase in the value of peripheral airway resistance R5-R20 compared to children born AGA. In children born with a BW <1500 g airway impedance Z and airway resistance at 5 Hz were inversely related to BW z-score. Respiratory symptoms were reported by almost one third of our population.
The impairment of lung function in children born preterm is a well-known finding. Over the years, longitudinal studies demonstrated significant reduction of FEV1 in cohorts of children born extremely preterm, especially in those with BPD [5,7,8,18,19], and also in children born late preterm [20]. Anomalies in airway elastic properties and resistance have been assessed in preterm children by different techniques such as the interrupter resistance Rint [21,22], the forced oscillation technique (FOT) [7,23-26] and IOS [20,26-30].
One study conducted in a group of 6-8 years old Swedish children born preterm with a BW <1500 g showed that, even if asymptomatic, those with BPD had worse IOS parameters with higher airway resistance and more negative reactance compared to children without BPD [28]. Same results were obtained in a similar cohort of 49 Finnish children studied at the age of 5-10 years [29]. Findings in adolescents born preterm between 24 and 31 weeks of gestation confirmed that those with a diagnosis of BPD had higher R5-R20 compared to peers without BPD26. In all the three studies FEV1 was lower and often impaired in subjects with BPD. Thunqvist et al. found in 151 extremely preterm children aged 6 years a higher airway resistance R5-R20 compared to controls born at term irrespective of a diagnosis of BPD [20]. Compared to controls born at term, greater airway resistance was described in young children born late preterm (34-36 weeks of gestation) and higher values of R5, R5-R20 and AX were reported in male adolescents born moderate to late preterm (32-36 weeks of gestation) [30] suggesting that also slight prematurity can have a negative effect on pulmonary function.
Our results are in line with these previous findings with a certain degree of respiratory impairment found in 7-9-year-old children born preterm. The IOS findings of increased resistance of central and peripheral airways, lower reactance and increased AX and Fres suggest that children born preterm may have an increase in airway tone particularly in peripheral airways. Imaging studies performed in school children born very preterm revealed structural lung abnormalities of the peripheral airways such as airway wall thickening, increased subpleural opacities (that usually stand for alveolar septal fibrosis), mosaic perfusion and air trapping both in children with BPD [31] and in children without BPD [8] suggesting a fixed peripheral airway narrowing [32]. These changes might be present especially in children who might have suffered from anatomical damages during fetal life like those with intrauterine growth restriction (IUGR).
IUGR is commonly suspected when BW is classified as SGA. IUGR is often the result of nutritional fetal deficiency and impaired oxygenation due to placental insufficiency [33] that usually manifests in the last phases of gestation when pulmonary parenchyma encounters acinar and alveolar phases. As a result, the growth of distal airways is the portion of the lung mainly affected as confirmed in animal studies [33]. Our population included 12 children born SGA and 8 of these were born moderate to late preterm. The mean difference between observed and predicted values of R5-R20 was higher in children born SGA, suggesting a possible greater impairment in small airway resistance in these children compared to children born AGA. In the study by Thunqvist et al. children born SGA had higher values of R5-R20 than those born AGA while no difference was noted in spirometry [20]. Similarly, Greenough et al. found higher airway resistance measured by pletismography in children born preterm (23-25 wks) and SGA [34]. Furthermore, in children with a VLBW (<1500 g) we found that airway impedance Z and airway resistance R5 negatively correlated with BW z-score and that in the multivariable linear regression analysis BW z-score was an independent predictor for Z5, R5 and AX values. The relationship between low BW and impaired lung function has been extensively studied in infants [34,35], childhood [5,8,36,37] and adults [38]. Our data may further confirm the association between fetal and airway growth, particularly in children with VLBW.
We speculate that the finding of lower FEF25-75 in our cohort may reflect the impairment of small airways demonstrated with the increased values of the IOS indices. Previous studies showed concordant information between the oscillometric method and spirometry [26,28,29] but IOS is considered to be more sensitive in evaluating peripheral airways [13]. However, while we found a significant lower FEV1 in subjects with BPD compared to those without BPD (-0.93 z-score), we did not find a significant difference with IOS. However, all IOS indices were slightly but not significantly higher in children with BPD. In our population children born extremely preterm were only 9.2% of total while more than half (51.9%) was born moderate to late preterm; 10 children were diagnosed with BPD because exposed to long-term oxygen therapy. These data point out that the number of subjects at risk for severe respiratory complications was small and that the not significant data obtained from IOS parameters when comparting children with and without BPD may be due to the small size of the sample. However, a recent prospective cohort study based in Italy on a large population of very preterm children (n 194) also reported no significant difference in airway resistance in subjects with and without BPD [22]. Regardless a diagnosis of BPD, preterm birth alone, interrupting the last phases of lung development, may have contributed to the abnormalities observed in our study in lung function tests.
Almost one third of children in our cohort reported at least one episode of wheezing confirming the higher prevalence of respiratory symptoms in preterms with and without BPD [4,25,36]. However, in contrast with other authors27, we did not find higher airway resistance in children who had experienced wheezing in the previous year.
Our study has certain limitations. The cohort did not include a control population and we compared our IOS data with the published reference values of a Brasilian pediatric population. Our population cannot be considered representative of the total population of preterm children born in our area because we studied only those in regular follow-up in our respiratory clinic. Furthermore, extremely preterm children were only 5 out of 54 and we excluded from the study subjects with severe neurological impairment likely excluding the most severe cases. Our limited number of participants might have contributed to the non-significant associations between all the range of BW and the various IOS parameters because of power issues. The effect of bronchodilators on airway resistance was not evaluated, making it difficult to exclude that the higher resistance found in our study can be due to reversible airway obstruction. In addition, we did not assess lung function serially over time. Future studies need to be conducted in a prospective manner to confirm the role of IOS in describing peripheral airway resistance of children born preterm and SGA and confirm our results.
Conclusion
Children born preterm showed some evidence of impaired lung function (higher large and small airway resistance, more negative reactance, higher AX and Fres, lower FEF25- 75) irrespective of BPD and respiratory symptoms. Preterm birth alone interrupting last phases of lung development may contribute to structural alterations with possible long-term consequences. In children born <1500 g airway resistance may be related to BW z-score and being born SGA may affect the development of small airways. Our results suggest that IOS is a feasible and non-invasive method to assess lung function and add additional information to standard spirometry about respiratory function of children born preterm. Follow-up of this high risk population taking account of their neonatal history and identifying pulmonary function impairments is important to early recognise lung deficit and predict long-term respiratory outcome in childhood and adulthood. Determining which are the clinical implications of the functional abnormalities seen in these subjects and finding new practises to promote their health remain an important subject for future studies.
Declarations
Ethics approval
This retrospective study was approved by the ethical committee of Parma University Hospital (protocol number 732/2018/OSS*7AOUPR).
Consent for publication
Not applicable.
Availability of data and materials
The dataset analysed in the current study is available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Funding
None.
Authors’ contribution
VF developed the design of the work, analysed the data and wrote the manuscript; IB, ES and MC collected the data and contributed to the analysis; CM, AC and GP gave a substantial scientific contribution in interpretation of data and critically reviewed the manuscript. All authors approved the final version of the manuscript.
Acknowledgements
We would like to thank Dr. Enrico Lombardi for the insightful suggestions.
References
March of Dimes, PMNCH, Save the Children, WHO (2012) Born Too Soon: The Global Action Report on Preterm Birth. World Health Organization. Geneva.[ Ref ]
Ronconi A, Corchia C, Bellù R, Gagliardi L, Mosca F, et al. (2011) Esiti dei neonati di basso peso nelle Terapie Intensive Neonatali partecipanti all’Italian Neonatal Network nel 2008. Roma: Istituto Superiore di Sanità. Rapporti ISTISAN 11/44.[ Ref ]
Narayanan M, Beardsmore CS, Owers-Bradley J, Dogaru CM, Mada M, et al. (2013) Catch-up alveolarization in ex-preterm children: evidence from (3)He magnetic resonance. Am J Respir Crit Care Med 187: 1104-1109.[ Ref ]
Sonnenschein-van der Voort AM, Arends LR, de Jongste JC, AnnesiMaesano I, Arshad SH, et al. (2014) Preterm birth, infant weight gain, and childhood asthma risk: a meta-analysis of 147,000 European children. J Allergy Clin Immunol 133: 1317-1329.[ Ref ]
Fawke J, Lum S, Kirkby J, Hennessy E, Marlow N, et al. (2010) Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med 182: 237-245.[ Ref ]
Kotecha SJ, Edwards MO, Watkins WJ, Henderson AJ, Paranjothy S, et al. (2013) Effect of preterm birth on later FEV1: a systematic review and meta-analysis. Thorax 68: 760-766.[ Ref ]
Verheggen M, Wilson AC, Pillow JJ, Stick SM, Hall GL (2016) Respiratory function and symptoms in young preterm children in the contemporary era. Pediatr Pulmonol 51: 1347-1355.[ Ref ]
Simpson SJ, Logie KM, O’Dea CA, Banton GL, Murray C, et al. (2017) Altered lung structure and function in mid-childhood survivors of very preterm birth. Thorax 72: 702-711.[ Ref ]
Saarenpää HK, Tikanmäki M, Sipola-Leppänen M, Hovi P, Wehkalampi K, et al. (2015) Lung Function in Very Low Birth Weight Adults. Pediatrics 136: 642-650.[ Ref ]
Vollsæter M, Røksund OD, Eide GE, Markestad T, Halvorsen T (2013) Lung function after preterm birth: development from mid-childhood to adulthood. Thorax 68: 767-776.[ Ref ]
Alan HJ, Eduardo B (2001) Bronchopulmonary Dysplasia. Am J Respir Crit Care Med 163: 1723-1729.[ Ref ]
de Assumpção MS, Gonçalves RM, Martins R, Bobbio TG, Schivinski CI (2016) Reference Equations for Impulse Oscillometry System Parameters in Healthy Brazilian Children and Adolescents. Respir Care 61: 1090-1099.[ Ref ]
Goldman MD, Saadeh C, Ross D (2005) Clinical applications of forced oscillation to assess peripheral airway function. Respir Physiol Neurobiol 148: 179-194.[ Ref ]
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. (2005) Standardisation of spirometry. Eur Respir J 26: 319-338.[ Ref ]
Quanjer PH, Stanojevic S, Cole TJ, ERS Global Lung Function Initiative (2012) Multi-ethnic reference values for spirometry for the 3-95-yr age range: the Global Lung Function 2012 equations. Eur Respir J 40: 1324- 1343.[ Ref ]
Asher MI, Keil U, Anderson HR, Beasley R, Crane J, et al. (1995) International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J 8: 483-491.[ Ref ]
Bertino E, Spada E, Occhi L, Coscia A, Giuliani F, et al. (2010) Neonatal anthropometric charts: the italian neonatal study compared with other european studies. JPGN 51: 353-356.[ Ref ]
Ronkainen E, Dunder T, Peltoniemi O, Kaukola T, Marttila R, et al. (2015) New BPD predicts lung function at school age: Follow-up study and meta-analysis. Pediatr Pulmonol 50: 1090-1098.[ Ref ]
Cazzato S, Ridolfi L, Bernardi F, Faldella G, Bertelli L (2013) Lung function outcome at school age in very low birth weight children. Pediatr Pulmonol 48: 830-837.[ Ref ]
Thunqvist P, Tufvesson E, Bjermer L, Winberg A, Fellman V, et al. (2018) Lung function after extremely preterm birth-A population-based cohort study (EXPRESS). Pediatr Pulmonol 53: 64-72.[ Ref ]
Kairamkonda VR, Richardson J, Subhedar N, Bridge PD, Shaw NJ (2008) Lung function measurement in prematurely born preschool children with and without chronic lung disease. J Perinatol 28: 199-204.[ Ref ]
Lombardi E, Fainardi V, Calogero C, Puglia M, Voller F, et al. (2018) Lung function in a cohort of 5-year-old children born very preterm. Pediatr Pulmonol 53: 1633-1639.[ Ref ]
Duiverman EJ1, Den Boer JA, Roorda RJ, Rooyackers CM, Valstar M, et al. (1988) Lung function and bronchial responsiveness measured by forced oscillometry after bronchopulmonary dysplasia. Arch Dis Child 63(7 Spec No): 727-732.[ Ref ]
Udomittipong K1, Sly PD, Patterson HJ, Gangell CL, Stick SM, et al. (2008) Forced oscillations in the clinical setting in young children with neonatal lung disease. Eur Respir J 31: 1292-1299.[ Ref ]
Vrijlandt EJ, Boezen HM, Gerritsen J, Stremmelaar EF, Duiverman EJ (2007) Respiratory health in prematurely born preschool children with and without bronchopulmonary dysplasia. J Pediatr 150: 256-261.[ Ref ]
Um-Bergström P, Hallberg J, Thunqvist P, Berggren-Broström E, Anderson M, et al. (2017) Lung function development after preterm birth in relation to severity of Bronchopulmonary dysplasia. BMC Pulm Med 17: 97.[ Ref ]
Er I, Gunlemez A, Uyan ZS, Aydogan M, Oruc M, et al. (2016) Evaluation of lung function on impulse oscillometry in preschool children born late preterm. Pediatr Int 58: 274-278[ Ref ]
Broström EB, Thunqvist P, Adenfelt G, Borling E, Katz-Salamon M (2010) Obstructive lung disease in children with mild to severe BPD. Respir Med 104: 362-370.[ Ref ]
.Malmberg LP, Mieskonen S, Pelkonen A, Kari A, Sovijärvi AR, et al. (2000) Lung function measured by the oscillometric method in prematurely born children with chronic lung disease. Eur Respir J 16: 598-603.[ Ref ]
Thunqvist P, Gustafsson PM, Schultz ES, Bellander T, Berggren-Broström E, et al. (2016) Lung Function at 8 and 16 Years After Moderate-to-Late Preterm Birth: A Prospective Cohort Study. Pediatrics 137: e20152056.[ Ref ]
Ronkainen E1, Perhomaa M, Mattila L, Hallman M, Dunder T (2018) Structural Pulmonary Abnormalities Still Evident in Schoolchildren with New Bronchopulmonary Dysplasia. Neonatology 113: 122-130.[ Ref ]
Reyburn B1, Martin RJ, Prakash YS, MacFarlane PM (2012) Mechanisms of injury to the preterm lung and airway: implications for long-term pulmonary outcome. Neonatology 101: 345-352.[ Ref ]
Harding R, Maritz G (2012) Maternal and fetal origins of lung disease in adulthood. Semin Fetal Neonatal Med 17: 67-72.[ Ref ]
Greenough A, Yuksel B, Cheeseman P (2004) Effect of in utero growth retardation on lung function at follow-up of prematurely born infants. Eur Respir J 24: 731-733.[ Ref ]
Dezateux C, Lum S, Hoo AF, Hawdon J, Costeloe K, et al. (2004) Low birth weight for gestation and airway function in infancy: exploring the fetal origins hypothesis. Thorax 59: 60-66.[ Ref ]
.Rona RJ, Gulliford MC, Chinn S (1993) Effects of prematurity and intrauterine growth on respiratory health and lung function in childhood. BMJ 306: 817-820.[ Ref ]
Anand D1, Stevenson CJ, West CR, Pharoah PO (2003) Lung function and respiratory health in adolescents of very low birth weight. Arch Dis Child 88: 135-138.[ Ref ]
Canoy D, Pekkanen J, Elliott P, Pouta A, Laitinen J, et al. (2007) Early growth and adult respiratory function in men and women followed from the fetal period to adulthood. Thorax 62: 396-402.[ Ref ]