Journal Name: Journal of Pediatrics and Infants
Article Type: Research
Received date: 27 October, 2017
Accepted date: 13 November, 2017
Published date: 20 November, 2017
Citation: El-Gamasy MA, Mehrez MM, Talaat K, Abo- Hagar H, Barr MA (2017) Trans-Esophageal Versus Conventional Hemodynamic Monitoring as Predictors of Responsiveness of Mechanically Ventilated Children to Fluid Therapy. J Pediat Infants. Vol: 1, Issu: 1. (01-08).
Copyright: © 2017 El-Gamasy MA, et al. This is an openaccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Mechanical ventilation has negative effect on the hemodynamic (HD) status of patients, which require boluses intravenous fluid (IVF). So Hemodynamic monitoring is indicated to help adjusting adequate fluid balance to maintain tissue perfusion.
Objective: To evaluate the hemodynamic parameters after fluid resuscitation in mechanically ventilated children.
Patients: 64 mechanically ventilated children in Pediatric Intensive Care Unit (PICU) received bolus IVF. They were divided in Group I as Responder and Group II as Non- responder to IVF.
Methods: Conventional monitoring including heart rate(HR), mean arterial blood pressure (MABP)and central venous pressure (CVP), Trans-esophageal measurement of Stroke Volume(SV), Cardiac Output (COP), Cardiac Index(CI), Systemic vascular resistance(SVR), Flow Time Corrected (FTc) and Systemic vascular resistance index(SVRI) before and 20 minutes after intravenous fluid bolus
Results: There was statistically non- significant difference in HR, MABP, CVP and Systemic Vascular Resistance SVR between group I compared with group II before and after IVS infusion also inside each group. SV, CI and FTc were significantly increased in group I after IVS infusion compared with before IVS infusion, also in group I compared with group II after IVS infusion. CVP, SV and SVRI had significant cutoff values to Predict length of stay (LOS) > 7 days.
Conclusion: Trans-esophageal monitoring for cardiac function could show the changes in hemodynamics after intravenous fluids better than conventional monitoring. (mainly in responders and to less instinct in non-responders).
Keywords
Hemodynamic; trans-esophageal; Cardiac Index; Systemic vascular resistance.
Abstract
Background: Mechanical ventilation has negative effect on the hemodynamic (HD) status of patients, which require boluses intravenous fluid (IVF). So Hemodynamic monitoring is indicated to help adjusting adequate fluid balance to maintain tissue perfusion.
Objective: To evaluate the hemodynamic parameters after fluid resuscitation in mechanically ventilated children.
Patients: 64 mechanically ventilated children in Pediatric Intensive Care Unit (PICU) received bolus IVF. They were divided in Group I as Responder and Group II as Non- responder to IVF.
Methods: Conventional monitoring including heart rate(HR), mean arterial blood pressure (MABP)and central venous pressure (CVP), Trans-esophageal measurement of Stroke Volume(SV), Cardiac Output (COP), Cardiac Index(CI), Systemic vascular resistance(SVR), Flow Time Corrected (FTc) and Systemic vascular resistance index(SVRI) before and 20 minutes after intravenous fluid bolus
Results: There was statistically non- significant difference in HR, MABP, CVP and Systemic Vascular Resistance SVR between group I compared with group II before and after IVS infusion also inside each group. SV, CI and FTc were significantly increased in group I after IVS infusion compared with before IVS infusion, also in group I compared with group II after IVS infusion. CVP, SV and SVRI had significant cutoff values to Predict length of stay (LOS) > 7 days.
Conclusion: Trans-esophageal monitoring for cardiac function could show the changes in hemodynamics after intravenous fluids better than conventional monitoring. (mainly in responders and to less instinct in non-responders).
Keywords
Hemodynamic; trans-esophageal; Cardiac Index; Systemic vascular resistance.
Introduction
Mechanical ventilation (MV) induces changes in intrapleural or intrathoracic pressure and lung volume which can affect the cardiovascular performance[1,2]. Daily fluid balance is used as predictor of outcome in mechanically ventilated patients; Positive fluid balance (PFB) contributes to increased mortality[3]. However reduction of intravascular volume due to negative fluid balance along with reduced venous return (VR) during positive pressure inspiration; results in a fall in stroke volume, which is initially compensated for by an increase in heart rate thereby maintaining cardiac output[4]. Meanwhile, with further volume depletion cardiac output and then blood pressure falls. This is associated with a reduction in organ perfusion[5]. Fluid therapy is considered the first step in the resuscitation in these patients as the use of vasopressor agents may increase organ hypoperfusion and ischemia[6]. HD monitoring may be used to estimate the physiological reserve that may in turn direct treatment and is indicated to alert about impending cardiovascular crisis before organ injury ensues[7]. Also to allow to monitor the response to fluid therapy[8].
Classical HD monitoring is based on the invasive measurement of systemic and pulmonary arterial and venous pressures; however, they have many potential flaws and complications[9]. Esophageal Doppler monitor is used in assessing CO and intravascular fluid status through using single-use probe, which is placed in the esophagus via the mouth or nose such monitoring helps in assessment of the initial hemodynamic state and judging response to therapy[3]. Its advantages include ease of use and absence of complications that can be associated with other more invasive methods of determining CO. also it allows continuous monitoring, so effects of fluid and inotropic therapy to be observed immediately, facilitating optimum titration of therapy[10]. In this study we will try to investigate hemodynamic changes following fluid resuscitation in mechanically ventilated children using transoesophogeal monitoring.
Methods
Sample size and inclusion criteria
This study was conducted in PICU of Pediatric department of Tanta university hospital, Egypt from September 2016 to September 2017 on 32 children; their ages ranged from 4-113 months, 20 males and 44 females. Patients were included in the study were mechanically ventilated patients who needed resuscitation with intravenous fluids with hemodynamic instability manifested clinically by Tachycardia and Tachypnea[11]. Signs of impaired organ perfusion including decreased urine output and altered mental status or signs of delayed peripheral perfusion including weak peripheral pulses, delayed capillary refill >2 sec and cool extremities, Temperature instability (hyperthermia, hypothermia) or Hypotension. The study has been approved by the local institutional research ethics committee.
Exclusion criteria
Patients who have Multiple Organ System Failure (MOSF), Complex congenital heart disease (CHD)or Patients with tracheo-oesophageal fistula(TOF) were excluded from the study.All patients were subject to transesophageal monitoring before and 20 minutes after intravenous fluid.
Protocol of fluid resuscitation: After confirming a five-min period of HD reading stability (arterial blood pressure and HR) Inspiratory Pressure(PIP) was adjusted to obtain an expiratory tidal volume of 6 mL/kg, Positive End Expiratory Pressure(PEEP) was not applied; all variables were measured before fluid loading[12].
Volume expansion was conducted by central line, administering 20 mL/ kg of normal saline for over 10 min, and then all variables were re-measured 20 minutes later. The subjects were divided into two groups according to the response to fluid infusion. Group I: Responder to IVS infusion and Group II Non- responder to IVS infusion. If SVI increased > 10% and/or SV increased ≥ 15%, these patients were considered responder to IVF otherwise they were considered non-responders[12].
The diagnosis of fluid refractory shock is considered if there is no response after infusion of 60 mL/kg isotonic saline “20 mL X 3 doses” (after that dopamine was given in cold shock and if no response gives epinephrine. Norepinephrine was given in warm shock). However, due to patient safety consideration, time factor and for research reasons, non-responders were considered the after 20 mL/ kg isotonic saline (single dose) [13]. On enrollment in the study, scoring systems for patients were used, Pediatric Risk for Mortality (PRISM) III score immediately on admission [14]and Sequential Organ Failure Assessment (SOFA) score 48 hours after admission. [15]Hemodynamic monitoring of stroke volume, cardiac output, cardiac index, systemic vascular resistance and systemic vascular resistance index by Deltex the CardioQTM Transesophageal Doppler. Cardio QTM Product code. (9015-7103); Deltex medical[16].
Insertion of the probe: Water-based lubricant was applied to probe tip and lower part of probe at insertion time. Oral placement of probe was adjusted, pushed probe was advanced until incisors were at the second depth marker at starting. No aggressive force was used. When the Cardio Q signal was located (descending aortic signal), the volume knob was adjusted as required. The selected CO parameters were measured continuously and saved to hard disk. When hemodynamically stable, 3 consecutive ODM recordings were registered, and the mean was calculated and plotted against each patient[17].
Statistical analysis
The collected data were organized, tabulated and statistically analyzed using SPSS version 19 (Statistical Package for Social Studies) created by IBM, Illinois, Chicago, USA. For numerical values the range mean and standard deviations were calculated[18]. The differences between two mean values before and after therapy were tested using paired student’s t test. Testing of mean differences between survivors and survivors was done using Mann-Whitney test (Z) due to small sample size in each category. For categorical variable the number and percentage were calculated. The level of significant was adopted at p < 0.05. A receiver operating characteristic (ROC) curve: used to illustrate the diagnostic properties of a test on a numerical scale[18].
Results
Children who were studied were divided into two groups Group I: Responder to IVS infusion and Group II: Nonresponder to IVS infusion. Table 1 shows demographic and clinical characteristics of the studied subjects. They were 64 patents with mean age 36.35 ± (24.96) months old were included. Table 2 showed that regarding PRISM III, in Relation to mortality, there was no statistically significant difference between studied groups (p >0.05). Table 3 compared between the studied groups as regard conventional and transoesophageal HD mointering, that there was no statistically significant difference between group I compared with group II before nor after IVS infusion as regard conventional HD monitoring including HR, MABP, CVP and SVR (p >0.05). There was also no statistically significant difference inside each group. (p >0.05).
Table1: Demographic and clinical characteristics of the studied subjects.
| Age (month) | |
|---|---|
| Range | 4-113 |
| Mean ± (SD) | 36.35 ± (24.96) |
| Gender (M/F) | 20/44 |
| BSA(M2) | |
| Range | 0.2-1.4 |
| Mean ± (SD) | 0.57 ± (0.36) |
| Diagnosis | Number |
| Bronchopneumonia, Respiratory failure | 6 |
| Metabolic disease (Refsum disease) | 4 |
| Convulsion, Metabolic disturbance | 2 |
| Status epilepticus | 6 |
| GullianBaririe with post meningitic hydrocephalus | 4 |
| Congenital Myopathy on MV | 2 |
| Intraventricular hemorrhage | 2 |
| Encephalitis | 4 |
| Infective endocarditis, cerebral infarction | 2 |
| Werdnig Hoffmann Syndrome | 4 |
| Congenital heart disease complicated with bronchopneumonia | 8 |
| 8 Sever persistent asthma complicated by bronchopneumonia | 6 |
| Complete atrioventricular canal with respiratory failure | 2 |
| Systemic lupus eryhematosus with shock | 4 |
| Down syndrome with bronchopneumonia & congestive heart failure | 2 |
| Cardiomyopathy complicated by cardiogenic shock | 4 |
| Myocarditis, dilated cardiomyopathy, arrhythmia | 2 |
| Myocarditis, dilated cardiomyopathy, arrhythmia | |
Table2: PRISM III in studied groups.
| PRISM III | Group I | Group II | ||
|---|---|---|---|---|
| Survived No. (%) | Died No. (%) | Survived No. (%) | Died No. (%) | |
| <10 | 5 (45.5%) | 2 (22.2%) | 3 (37.5%) | 2 (50%) |
| 10 – 20 | 5 (45.5) | 3 (33.3%) | 4(50%) | 1(25%) |
| >20 | 1 (9%) | 4 (44.4%) | 1 (12.5 %) | 1 (25%) |
| >χ2 | 4.602 | |||
| >MCp | 0.651 | |||
| χ2: Chi square test MC: Monte Carlo test |
||||
Regarding Flow Time Corrected
There was statistically significant increase in group I after IVS infusion compared with before IVS infusion (p < 0.05). Otherwise, there was no statistically significant difference between studied groups. (p >0.05).
Regarding Stroke volume and SVI
There was statistically significant increase in group I after IVS infusion compared with before IVS infusion. There was statistically significant increase in group I compared with group II after IVS infusion. (p < 0.05). Otherwise, there was no statistically significant difference between studied groups. (p > 0.05).Table 3 showed also that regarding to Cardiac Index: There was statistically significant increase in group I and group II after IVS infusion compared with before IVS infusion (p < 0.05). Otherwise, there was no statistically significant difference between studied groups. (p < 0.05).
Table3: Comparison between conventional and transoesophageal HD mointering between the studied groups.
| Items | Mean ± SD | Paired t test | |||
|---|---|---|---|---|---|
| t | p1 | ||||
| HR | Group I | Before | 125.25 ± 14.30 | 1.92 | 0.069 |
| After | 117.33 ± 18.78 | ||||
| Group II | Before | 127.6 ± 15.68 | 0.79 | 0.446 | After | 124.75 ± 21.39 |
| MABP | Group I | Before | 75 ± 18.5 | 0.76 | 0.45 |
| After | 78.4 ± 19.7 | ||||
| Group II | Before | 68.7 ± 13.8 | 0.043 | 0.96 | After | 168.9 ± 15.1 |
| CVP | Group I | Before | 6.8 ± 2.93 | 0.82 | 0.42 |
| After | 6.95 ± 3.11 | ||||
| Group II | Before | 7.16 ± 4.19 | 0.22 | 0.82 | After | 7.45 ± 5.1 |
| SV | Group I | Before | 9.74 ± 6.73 | 6.57 | <0.00001 |
| After | 13.28 ± 8.14 | ||||
| Group II | Before | 8.55 ± 6.73 | 1.78 | 0.101 | After | 8.86 ± 4.60 |
| SVI | Group I | Before | 16.68 ± 5.83 | 5.51 | <0.0056 |
| After | 23.61 ± 7.73 | ||||
| Group II | Before | 18.20 ± 6.14 | 1.28 | 0.2 | After | 18.41 ± 6 |
| FTc | Group I | Before | 295 ± 56.72 | 3.55 | <0.0020 |
| After | 332.4 ± 74.66 | ||||
| Group II | Before | 296.66 ± 59.71 | 1.15 | 0.27 | After | 315.83 ± 38.77 |
| CO | Group I | Before | 1.29 ± 0.86 | 6.4 | <0.00001 |
| After | 1.71 ± 1.05 | ||||
| Group II | Before | 0.93 ± 0.42 | 10.79 | <0.00001 | After | 1.14 ± 0.45 |
| CI | Group I | Before | 2.28 ± 0.68 | 5.5 | <0.0065 |
| After | 3.13 ± 0.99 | ||||
| Group II | Before | 2.11 ± 0.56 | 7.76 | <0.00001 | After | 2.62 ± 0.58 |
| SVR | Group I | Before | 6019 ± 5046 | -0.23 | 0.81 |
| After | 5688 ± 6501 | ||||
| Group II | Before | 6637 ± 4667 | -2.02 | 0.067 | After | 5114 ± 2436 |
| SVRI | Group I | Before | 2663 ± 1750 | -2.15 | 0.043 |
| After | 2021 ± 901 | ||||
| Group II | Before | 2519 ± 1196 | -2.05 | 0.064 | After | 2074 ± 760 |
p1: before versus before and after versus after in two groups;P2: before versus after in the same group;CO: Cardiac output;CVP: central venous pressure; SV: Stroke Volume;SVRI: Systemic Vascular Resistance Index.
Regarding Cardiac Output
There was statistically significant increase in group I and group II after IVS infusion compared with before IVS infusion. (p < 0.05). There was statistically significant increase in group I compared with group II after IVS infusion. (p < 0.05). Otherwise, there was no statistically significant difference between studied groups (p > 0.05).
Table 3 showed also that regarding to Cardiac Index: There was statistically significant increase in group I and group II after IVS infusion compared with before IVS infusion (p < 0.05). Otherwise, there was no statistically significant difference between studied groups. (p < 0.05).
Regarding Cardiac Output
There was statistically significant increase in group I and group II after IVS infusion compared with before IVS infusion. (p < 0.05). There was statistically significant increase in group I compared with group II after IVS infusion. (p < 0.05). Otherwise, there was no statistically significant difference between studied groups (p > 0.05).
Regarding Systemic Vascular Resistance Index
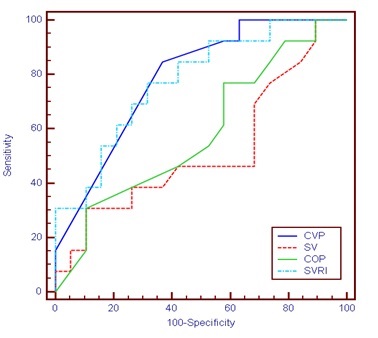
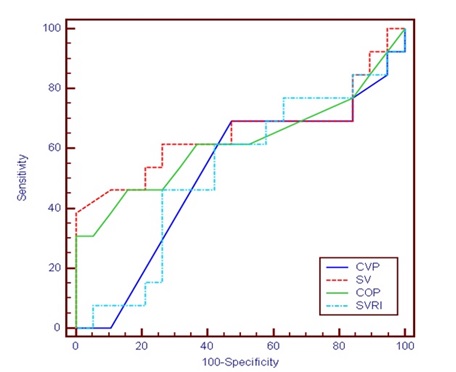
There was statistically significant decrease in group I after IVS infusion compared with before IVS infusion. Otherwise, there was no statistically significant difference between studied groups (p > 0.05).Table 4 and Figure 1 Showed that regarding ROC Curve for CVP, SV CO and SVRI before treatment to predict LOS >7 Days: The area under curve of CVP (0.787) was significant, cutoff value to Predict LOS >7 days was 5, (Sensitivity =84.62%, specificity =63.16 %, PPV= 61.11, NPV= 85.71, accuracy = 71.88%). The area under curve of SV (0.520) was non-significant, cutoff value to Predict LOS >7 days was 9.4, (sensitivity= 46.15%, specificity = 31.58%, PPV=31.58, NPV= 46.15, accuracy = 37.50%). The area under curve of COP (0.585) was non-significant, cutoff value to Predict LOS >7 days was 0.6., (sensitivity=30.77 %, specificity =89.47%, PPV= 66.67, NPV= 65.38, accuracy =65.63 %). The area under curve of SVRI (0.777) was significant, cutoff value to Predict LOS >7 days was 2288 (Sensitivity=76.92%, specificity = 68.42%, PPV= 62.50, NPV=81.25, accuracy = 71.88%). Table 5 and figure 2 showed that regarding ROC curve for CVP, SV CO and SVRI before treatment to predict mortality. The area under curve of CVP (0.516) was non-significant, cutoff value to Predication of mortality was 5, (Sensitivity =69.23%, specificity =52.63 %, PPV= 50, NPV= 71.43, accuracy = 59.38%). The area under curve of SV (0.651) was nonsignificant, cutoff value to Predication of mortality was 16 (sensitivity= 38.46%, specificity = 100%, PPV=100, NPV= 70.37, accuracy = 75%). The area under curve of COP (0.617) was non- significant, cutoff value to Predication of mortality was 1.8, (sensitivity=30.77 %, specificity =100%, PPV= 100, NPV= 67.86, accuracy =71.88 %). The area under curve of SVRI (0.526) was non- significant, cutoff value to Predication of mortality was 2473 (Sensitivity=46.15%, specificity = 73.68%, PPV= 54.55, NPV=66.67, accuracy = 62.50%).
Table4: ROC Curve For CVP, SV, COP and SVRI Before Treatment to Predict Length of Stay.
| AUC | P | Cut off | Sensitivity | Specificity | PPV | NPV | Accuracy | 95% CI | ||
|---|---|---|---|---|---|---|---|---|---|---|
| LL | UL | |||||||||
| CVP | 0.787* | <0.001* | 5 | 84.62 | 63.16 | 61.11 | 85.71 | 71.88 | 0.607 | 0.911 |
| SV | 0.520 | 0.856 | 9.4 | 46.15 | 31.58 | 31.58 | 46.15 | 37.50 | 0.337 | 0.699 |
| COP | 0.585 | 0.421 | 0.6 | 30.77 | 89.47 | 66.67 | 65.38 | 65.63 | 0.398 | 0.7 |
| SVRI | 0.777* | <0.001* | 2288 | 76.92 | 68.42 | 62.50 | 81.25 | 71.88 | 0.595 | 0.904 |
Figure 1: ROC Curve for Hemodynamic Parameters before Treatment to Predict Length of Stay.
Table5: ROC Curve For CVP, SV, COP and SVRI before treatment to predict mortality.
| Percentage of change | AUC | P | Cut off | Sensitivity | Specificity | PPV | NPV | Accuracy | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|---|
| LL | UL | |||||||||
| CVP | 0.516 | 0.876 | 5 | 69.23 | 52.63 | 50 | 71.43 | 59.38 | 0.333 | 0.695 |
| SV | 0.651 | 0.188 | 16 | 38.46 | 100 | 100 | 70.37 | 75 | 0.463 | 0.81 |
| COP | 0.617 | 0.307 | 1.8 | 30.77 | 100 | 100 | 67.86 | 71.88 | 0.429 | 0.782 |
| SVRI | 0.526 | 0.81 | 2473 | 46.15 | 73.68 | 54.55 | 66.67 | 62.5 | 0.342 | 0.704 |
AUC: Area Under Curve, 95% CI: Confidence interval, LOS: length of stay, CO: Cardiac output, CVP: central venous pressure, LL: Lower limit, NPV: Negative Predictive value, PPV: Positive Predictive Value, SV: Stroke Volume, SVRI: Systemic Vascular Resistance Index, UL: Upper limit. |
||||||||||
Figure 2: ROC Curve for Hemodynamic Parameters Before Treatment To Predict Mortality.
Discussion and Conclusion
Accurate assessment of a patient’s volume status is a critical task in the care of critically ill patients. Despite this, most decisions regarding fluid therapy are made either empirically or with limited and poor data[19]. There is controversial data regarding the protocol of fluid resuscitation as in some studies aggressive fluid therapy protocol targeted to CVP and physiological variables resulted in reduced organ failure and improved survival in patients with severe sepsis and septic shock[20]. However, later studies in critically ill patients have demonstrated that the conservative strategy of fluid management improved lung function and shortened the duration of mechanical ventilation and intensive care without increasing non pulmonary-organ failures[21]. When giving intravenous fluids, two questions are asked: first, what is the current hemodynamic status of the patient? Second, if he receives continued fluid resuscitation or a fluid bolus, will physiological variables improve? [22]. The present study showed that regarding HR MABP and there was no significant difference between studied groups during study period. This was in contrast with previous research [23] who found that there was significantly decreased HR and significant increase in MABP in responders to a rapidly administered IVS infusion in critically ill patients. However, this was in agreement in non-responders whose HR and MABP were non–significant. This may be explained by that in shock there was decrease in preload leading to decrease in SV, the HR increase as a compensatory mechanism to maintain CO, so giving IVS which increase intravascular volume and preload will lead to increased SV and CO and decreased HR[24]. Also administration IVS lead to increase intravascular osmolality and improve hemodynamic instability which causes increase in MABP[25]. As regards to CVP there was no significant difference between studied groups during study period.
This was in accordance with previous research[12], who found that there were no significant differences in CVP between studied groups. This may be explained by that volume status of patients who have shock and on MV cannot be accurately gauged and monitored by CVP because of the changes in ventricular compliance and disease, changes in thoracic and lung compliance, frequent use of positive pressure ventilation and pulmonary vascular disease[26]. The present study showed that regarding SV, there was significant increase after compared with before IVS infusion in responders and in responders compared with nonresponders after IVS infusion. This was in accordance with previous researches[27-29], who found that there was significantly increased in SV after fluid infusion. Also as regarding SVI there was significant increase after compared with before IVS infusion in responders and in responders compared with non- responders after IVS infusion. This was in accordance with previous research [30] who found that there was significantly increased in SVI after IVS infusion and used as good predictive of fluid responsiveness in sedated mechanically ventilated children after surgery. Also, this was in agreement in non-responders whose SVI was non–significant.
Both results may be explained by that intravenous infusion lead to increase intravascular volume, increase in left ventricular end-diastolic volume (LVEDV) and an increase in the left ventricular ejection fractions (LVEF) leading to increase SV and SVI[31]. The present study showed that regarding FTc there was significant increase after compared with before IVS infusion in responder. This is may be explained by that FTc is affected by left ventricular preload which improves by volume expansion[31]. This was in accordance with previous research [32] who found that there was increase in FTc after 12 min fluid infusion in responder group Also, this was in agreement in non-responders whose FTc was non–significant and with previous research [33] who found that there was increase in FTc in responders after fluid infusion. However, this was in contrast in nonresponders whose FTc was significantly increased in their study. Our results were in contrast with previous research [34] who found that there was no significant difference in FTc after intravenous fluid infusion between responders. However, this was in agreement in non-responders whose FTc was non–significant in their study.
The present study showed that regarding CO there was significant increase after compared with before IVS infusion in responders and non-responders. Also, in responders compared with non-responders after IVS infusion. This was in accordance with previous researches [35,36] who found that there was increase in CO in responders and non-responders after volume expansion. The present study showed that regarding CI, there was significant increase after compared with before IVS infusion in responders and non-responders. This was in accordance with previous research[35], who found that there was increase in CI in responders and non-responders. This may be explained by that IVS infusion lead to increased intravascular volume, which in turn increases preload and if blood pressure improves, hence better systemic perfusion. These designate improved CO and CI combined with less cardiac work (if associated with significantly lower HR)[24]. Our study showed that regarding SVR there was a decrease however it wasn’t statistically significant before and after and between studied groups during study period. This was in contrast with previous research [31] who found that there was significantly decrease in SVR after rapidly administered volume infusion. However, this was in agreement in nonresponders whose SVR was non–significant. This may be explained by the small number of patients in our study. Regarding SVRI was significantly decreased after compared with before IVS infusion in responders.
This was in accordance with previous research [31] In addition, this was in agreement in non-responders whose SVRI was non–significant. Likewise, Some authors [23] found that there was significantly decreased in SVRI in responder receiving resuscitation by IVS. This may be explained by that during shock the heart trying to increase CO to preserve adequate perfusion to vital tissue and to do that it must increase afterload (SVR) and after treatment of shocked patients by intravenous fluid leading to increase intravascular volume hence, decreased SVR and SVRI[24]. The prediction of LOS >7 days using ROC curve was significant to CVP and SVRI and non-significant SV and COP. Otherwise, the prediction of mortality using ROC curve was non-significant to (CVP, SV, COP, and SVRI). The conventional hemodynamic (HR, MABP and CVP) monitoring failed (nonsignificant difference) to illuminate the alterations occurred in the studied groups during study period. Nevertheless, advanced hemodynamic monitoring SV, SVI, FTc, CO, CI (significant increase) and SVRI (significant decrease) could show the changes (mainly in responders and to less instinct in non-responders (need further investigations). Limitations of the study Insertion and fixation of the probes need skills and close observation. Manipulating with the probes after each meal, suction or sudden movement to get correct reading.
1. Cournand A, Motley HL, Werko L, Richards DW (1948) Jr Physiological studies of the effects of intermittent positive pressure breathing on cardiac output in man. Am J Physiol 152: 162–74.
2. Michard F, Teboul JL(2000) Using heart lung interactions to assess fluid responsiveness during mechanical ventilation. Crit Care 4: 282–9.
3. Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, et al., (2008) Sepsis Occurrence in Acutely Ill Patients (SOAP) Investigators. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care 12: R74.
4. Michard F(2005) Changes in arterial pressure during mechanical ventilation. Anesthesiology 103: 419–28.
5. Murakawa K, Kobayashi A(1988) Effects of vasopressors on renal tissue gas tensions during hemorrhagic shock in dogs. Crit Care Med 16: 789–92.
6. Marik, P.E Monnet, X. &Teboul, JL. (2011) Hemodynamic parameters to guide fluid therapy Ann. Intensive Care 1: 1.
7. Pinsky MR (2014) Functional haemodynamic monitoring. Curr Open Crit Care 20: 288-93.
8. Brierley J, Carcillo JA, Choong K, Cornell T, Decaen A, et al., (2009) Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med 37: 666-88.
9. Bigatello LM, George E(2002) Hemodynamic monitoring. Minerva Anestesiol 68: 219-25.
10. King SL(2004) The Use of the Oesophageal Doppler Monitor in the Intensive Care Unit. Crit Care and Resusc6: 113-22.
11. Carcillo JA(2012) Capillary refill time is a very useful clinical sign in early recognition and treatment of very sick children. PediatrCrit Care Med 13: 210-2.
12. Byon HJ, Lim CW, Lee JH, Park YH, Kim HS, et al., (2013) Prediction of fluid responsiveness in mechanically ventilated children undergoing neurosurgery Br J Anaesth 110: 586-91.
13. Brierly J, Carcillo JA, Choong K, et al.,(2009) Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine, Crit Care Med 37: 666–88.
14. Pollack MM, Patel KM, Ruttimann UE(1996) PRISM III: An updated Pediatric Risk of Mortality score. Crit Care Med 24: 743-52.
15. Jones AE, Trzeciak S, Kline JA(2009) The sequential organ failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypo perfusion at the time of emergency department presentation. Crit Care Med 37: 1649-54.
16. Deltex Medical (2009) Deltex™, CardioQ™; trademarks of Deltex Medical.
17. King SL(2004) The Use of the Oesophageal Doppler Monitor in the Intensive Care Unit. Crit Care and Resusc 6: 113-22.
18. SPSS version 19 Statistical Package for Social Studies, SPSS Inc., Chicago,Il, USA.
19. Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, et al., (2006) Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 34: 344–53.
20. Rivers E, Nguyen B, Havstad S Ressler J, Muzzin A, Knoblich B, et al., (2001) Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345: 1368–77.
21. Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, et al.,(2006) Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354: 2564-75.
22. Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, et al., (2012) RENAL Replacement Therapy Study Investigators. An observational study fluid balance and patient outcomes in the Randomized Evaluation of Normal vs. Augmented Level of Replacement Therapy trial. Crit Care Med 12: 1753–60.
23. Robert Kraft, Herndon DN, Branski LK, Finnerty CC, Leonard KR, et al., (2013) Optimized fluid management improves outcomes of pediatric burn patients. J Surg Res 181: 121-8.
24. Epstein D, Randall CW(2006) Cardiovascular physiology and shock. Mosby Elsevier, Philadelphia, PA.
25. Frank m. van der sande, antinus j. luik, jeroen p. kooman, vicverstappen, and karelm.l. leunissen(2000) Effect of Intravenous Fluids on Blood Pressure Course during Hemodialysis in Hypotensive-Prone Patients JASN11: 550-5.
26. John H. Boyd, Jason Forbes, Taka-akiNakada, Keith R. Walley, James A.Russell(2011) Fluid Resuscitation in Septic ShockCrit Care Med 39: 259-65.
27. Maurizio Cecconi Anthony k. parsons and Andrew Rhodes (2011) What is a fluid challenge? Current Opinion in Critical Care 17: 290–5.
28. LamiaB, ochagaviaA, monnetX, chemalaD, richardC, et al.,(2007) Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity intensive Care Med 33: 1125-32.
29. Guinot P-G., J. Godart, B. de Broca, E. Bernard, E. Lorne, et al., (2015) Dupont Ability of stroke volume variation measured by oesophageal Doppler monitoring to predict fluid responsiveness during surgery. Clinical practice Br. J. Anaesth 114: 167-8.
30. Vergnaud E, Vidal C, Verchère J, Miatello J, Meyer P, et al., (2015) Stroke volume variation and indexed stroke volume measured using bioreactance predict fluid responsiveness in postoperative children. Br. J. Anaesth 114: 103-109.
31. Calvin JE, Driedger AA, Sibbald WJ(1981) The hemodynamic effect of rapid fluid infusion in critically ill patients. Surgery 90: 61-76.
32. Lee JH, Kim JT, Yoon SZ(2007) Evaluation of corrected flow time in oesphageal Doppler as a predictor of fluid responsiveness. British Journal of Anaesthesia 99: 343–8.
33. Monnet X, Rienzo M, Osman D(2005) Esophageal Doppler monitoring predicts fluid responsiveness in critically ill ventilated patients. Intensive Care Med 31: 1195–201.
34. Vallee F, Fourcade O, De Soyres O(2005) Stroke output variations calculated by esophageal Doppler is a reliable predictor of fluid response. Intensive Care Med 31: 1388–93.
35. Randa Aly Soliman, Shereif Samir, Ayman el Naggar, Khalaf El Dehely(2015) Stroke volume variation compared with pulse pressure variation and cardiac index changes for prediction of fluid responsiveness in mechanically ventilated patients. Critical Care Medicine Department.
36. Vikas Singh, Sudhanshu K Dwivedi, Sharad Chandra, RiteshSanguri, Rishi Sethi, et al., (2014) Optimal fluid amount for haemodynamic benefit in cardiac tamponade. Eur Heart J Acute Cardiovasc Care 3: 158-64.